Question: 2. Boiling point indicates the strength of intermolecular interactions. Given the boiling points in parentheses, rank the following molecules in order from strongest to weakest
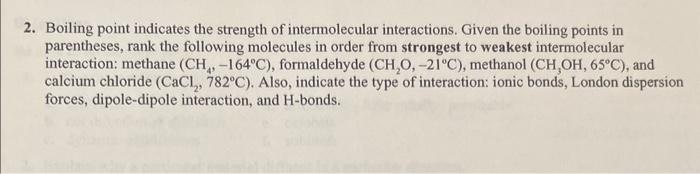
2. Boiling point indicates the strength of intermolecular interactions. Given the boiling points in parentheses, rank the following molecules in order from strongest to weakest intermolecular interaction: methane (CH4,164C), formaldehyde (CH2O,21C), methanol (CH3OH,65C), and calcium chloride (CaCl2,782C). Also, indicate the type of interaction: ionic bonds, London dispersion forces, dipole-dipole interaction, and H-bonds
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


