Question: 2 C 2 H 6 ( g ) + 7 O 2 ( g ) 4 C O 2 ( g ) + 6 H
In a certain experiment, and were added to a previously evacuated
reaction vessel. The reaction mixture was sparked and the reaction represented by the equation
shown above occurred until one of the reactants was completely consumed.
a Convert each quantity of reactant into mol
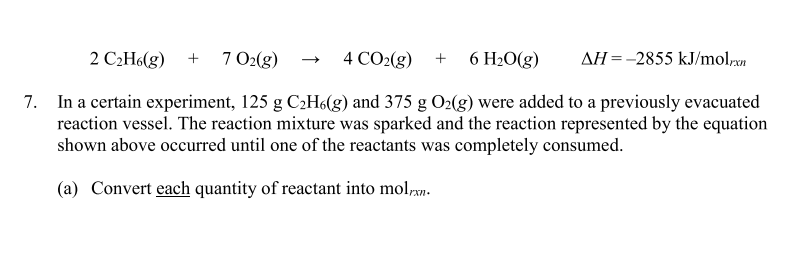
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


