Question: 2. Consider a continuous stirred tank reactor shown in FIGURE Q1. An irreversible exothermic reaction A+B takes place in the reactor. The system is cooled
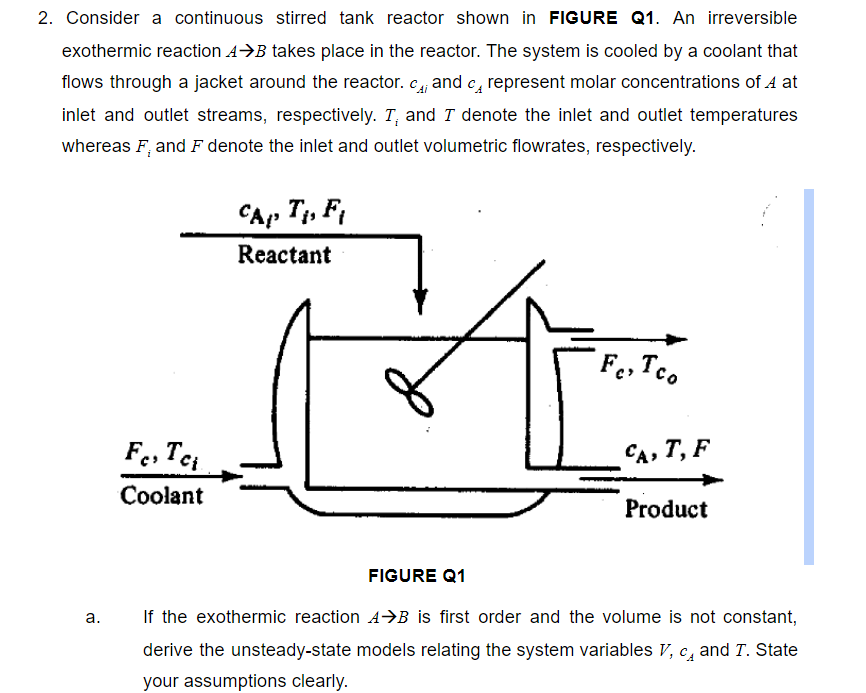
2. Consider a continuous stirred tank reactor shown in FIGURE Q1. An irreversible exothermic reaction A+B takes place in the reactor. The system is cooled by a coolant that flows through a jacket around the reactor. C.; and ca represent molar concentrations of A at inlet and outlet streams, respectively. T, and I denote the inlet and outlet temperatures whereas F, and F denote the inlet and outlet volumetric flowrates, respectively. CA, T, Fi Reactant Fe, Tco CA, T, F FeiTa Coolant Product FIGURE Q1 . a. If the exothermic reaction A+B is first order and the volume is not constant, derive the unsteady-state models relating the system variables V, c, and T. State your assumptions clearly
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


