Question: 2. Consider the reaction - A+BC The reaction is elementary; however, a zero order can assumed for B when CB0>10CA0. For the system being considered,
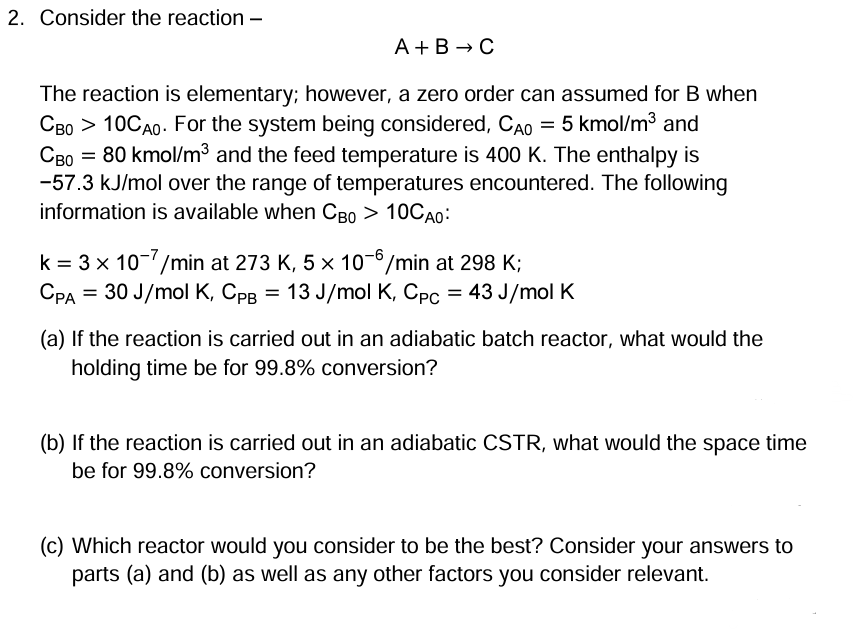
2. Consider the reaction - A+BC The reaction is elementary; however, a zero order can assumed for B when CB0>10CA0. For the system being considered, CA0=5kmol/m3 and CB0=80kmol/m3 and the feed temperature is 400K. The enthalpy is 57.3kJ/mol over the range of temperatures encountered. The following information is available when CB0>10CA0 : k=3107/minat273K,5106/minat298K;CPA=30J/molK,CPB=13J/molK,CPC=43J/molK (a) If the reaction is carried out in an adiabatic batch reactor, what would the holding time be for 99.8% conversion? (b) If the reaction is carried out in an adiabatic CSTR, what would the space time be for 99.8% conversion? (c) Which reactor would you consider to be the best? Consider your answers to parts (a) and (b) as well as any other factors you consider relevant. 2. Consider the reaction - A+BC The reaction is elementary; however, a zero order can assumed for B when CB0>10CA0. For the system being considered, CA0=5kmol/m3 and CB0=80kmol/m3 and the feed temperature is 400K. The enthalpy is 57.3kJ/mol over the range of temperatures encountered. The following information is available when CB0>10CA0 : k=3107/minat273K,5106/minat298K;CPA=30J/molK,CPB=13J/molK,CPC=43J/molK (a) If the reaction is carried out in an adiabatic batch reactor, what would the holding time be for 99.8% conversion? (b) If the reaction is carried out in an adiabatic CSTR, what would the space time be for 99.8% conversion? (c) Which reactor would you consider to be the best? Consider your answers to parts (a) and (b) as well as any other factors you consider relevant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


