Question: 2. Consider the reaction, for which Kc 0.10 at 2,000C. Starting with initial concentrations of 0.040 M of N2 and 0.040 M of O2,
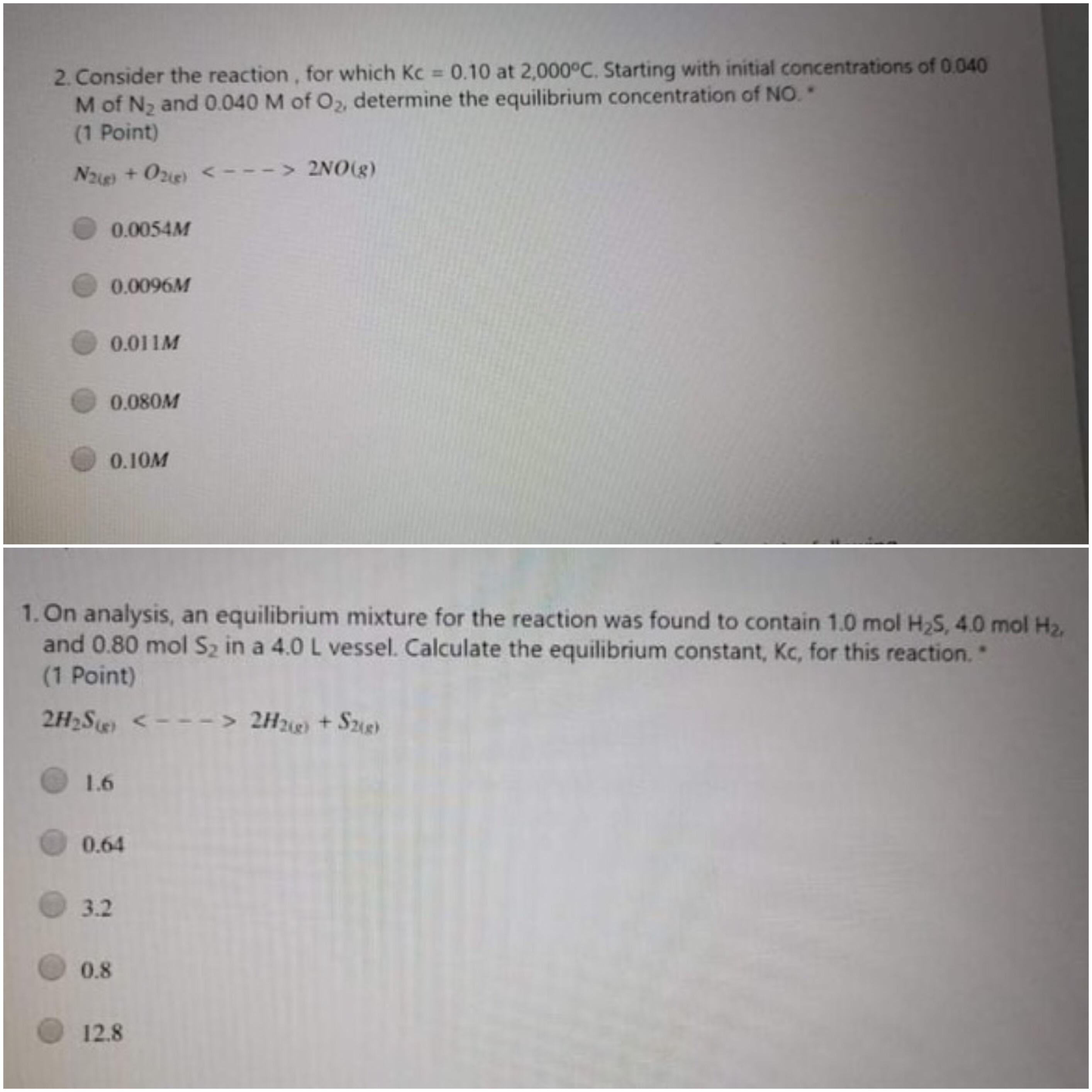
2. Consider the reaction, for which Kc 0.10 at 2,000C. Starting with initial concentrations of 0.040 M of N2 and 0.040 M of O2, determine the equilibrium concentration of NO. (1 Point) %3D Nae +O2ie) < ---> 2NO0(g) > 2NO(g) 0.0054M 0.0096M 0.011M 0.080M 0.10M 1. On analysis, an equilibrium mixture for the reaction was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol Sz in a 4.0 L vessel. Calculate the equilibrium constant, Kc, for this reaction. (1 Point) 2H2Se < ---> 2H2ie + Sze ) 1.6 0.64 3.2 0.8 12.8
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


