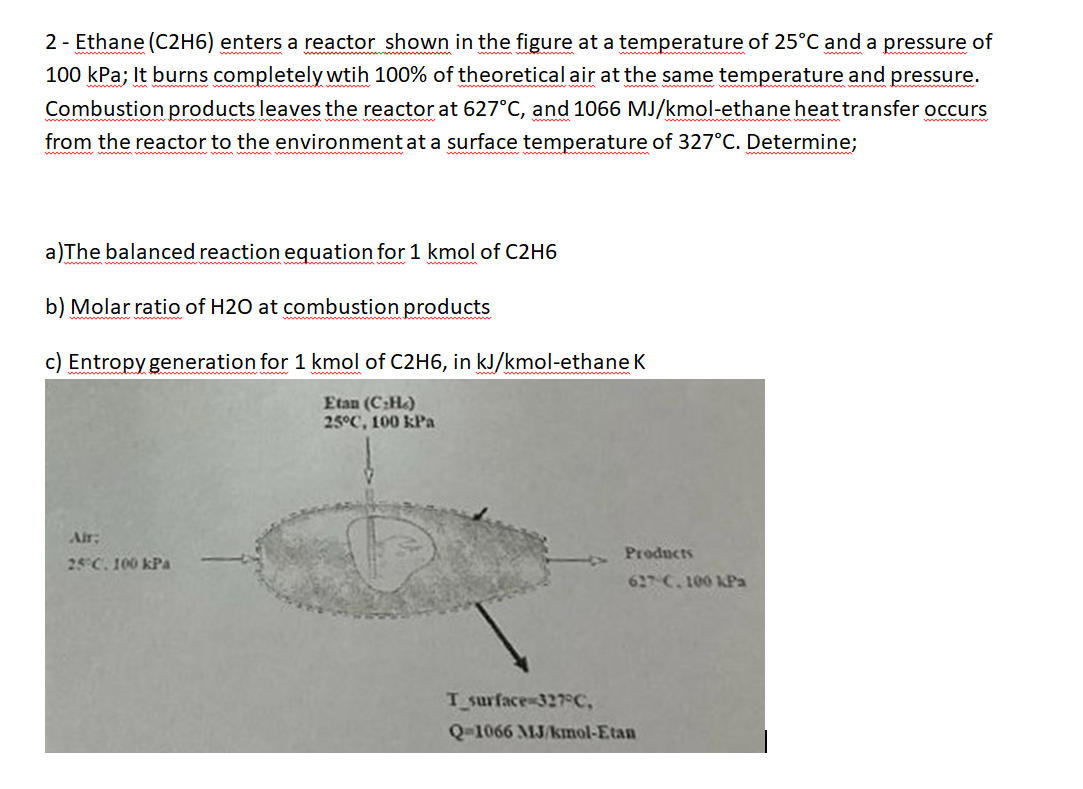
Question: 2 - Ethane ( C 2 H 6 ) enters a reactor shown in the figure at a temperature of 2 5 C and a
Ethane CH enters a reactor shown in the figure at a temperature of and a pressure of
kPa; It burns completely wtih of theoretical air at the same temperature and pressure.
Combustion products leaves the reactor at and molethane heat transfer occurs
from the reactor to the environment at a surface temperature of Determine;
aThe balanced reaction equation for kmol of
b Molar ratio of at combustion products
c Entropy generation for kmol of in molethane
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


