Question: 2 g NaCl 1 5 0 m % . mass / volume concentration 2 gNaC l 1 5 0 m L 1 0 0 =
NaCl
massvolume concentration
gNaC
Molarity
:
:
molNa
osmolarity
osmules
Is this correct
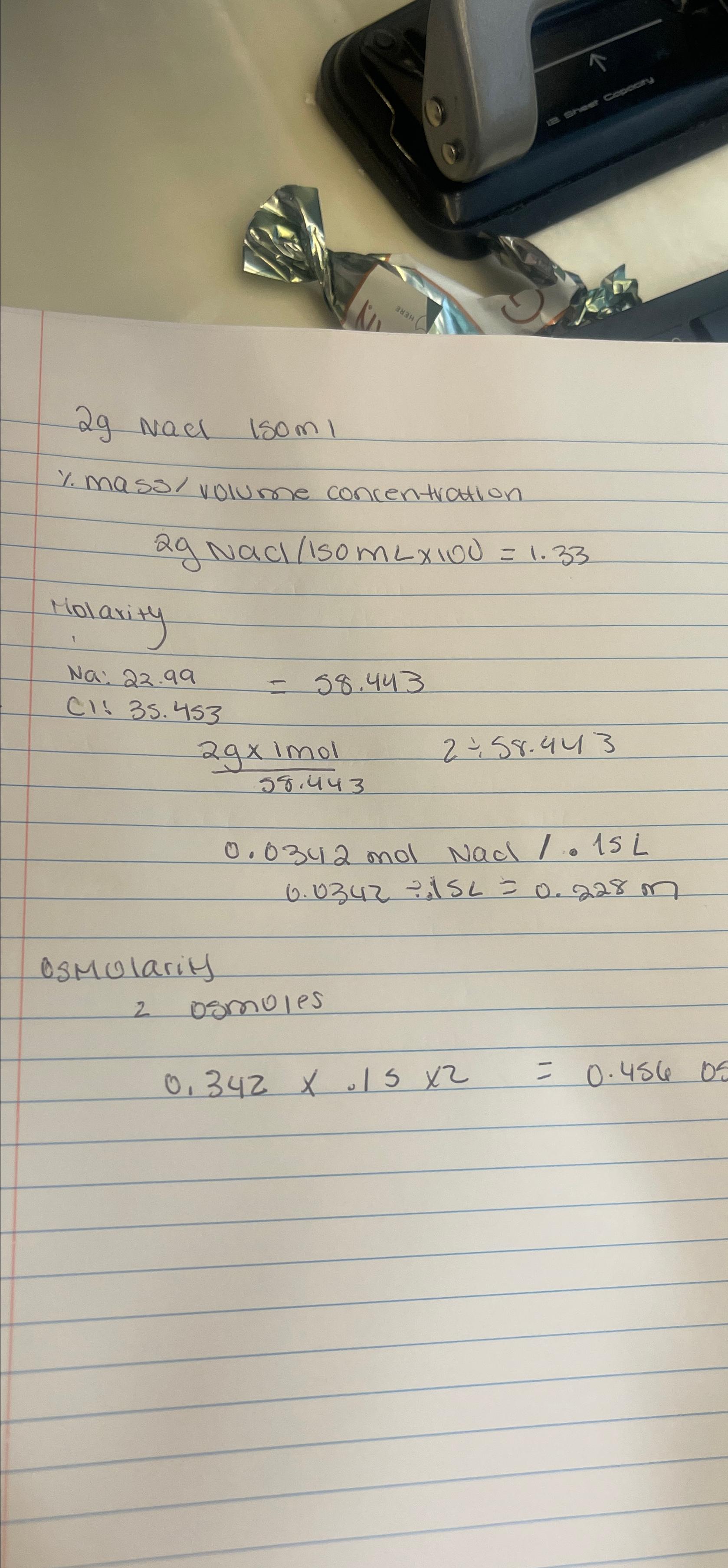
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


