Question: please help me to find a and b ? Method 1. To determine the concentration and the dissociation constant of the unknown acid solution (a)
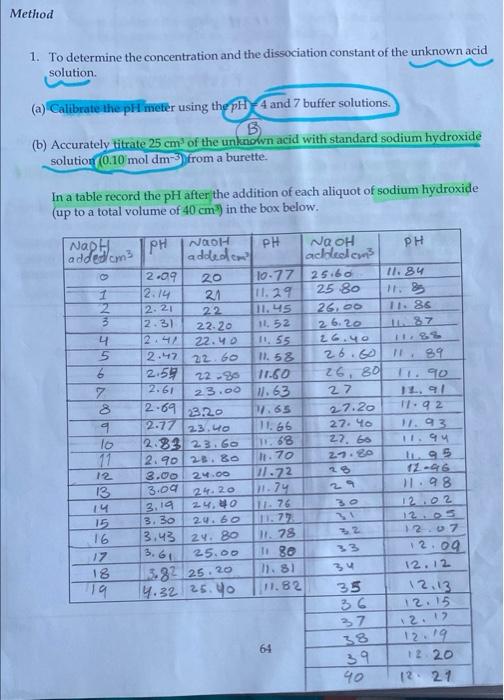
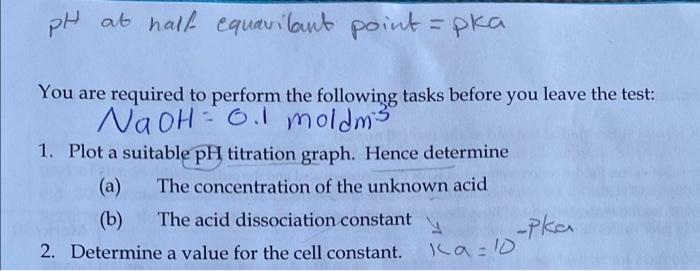
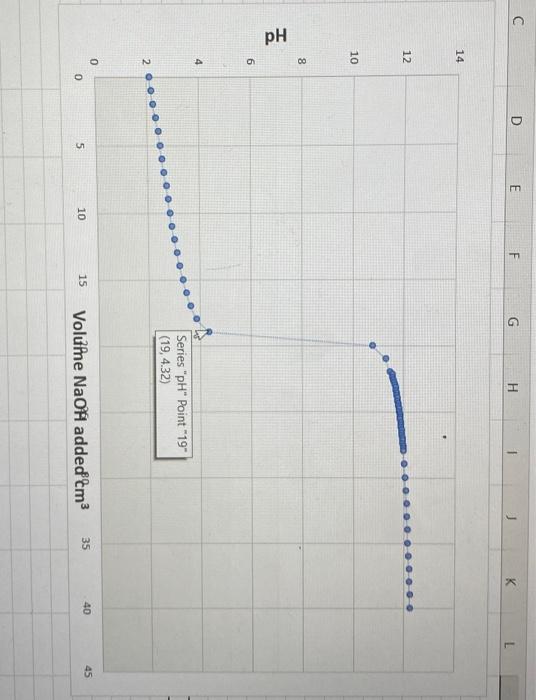
Method 1. To determine the concentration and the dissociation constant of the unknown acid solution (a) Calibrate the pH meter using the pH 4 and 7 buffer solutions B (b) Accurately titrate 25 cm of the unknown acid with standard sodium hydroxide solution 0.10 mol dm from a burette. In a table record the pH after the addition of each aliquot of sodium hydroxide (up to a total volume of 40 cm) in the box below. 8 NN ON Naph PH NOOH PH added cm3 addedcm 2.09 20 10.77 12.14 21 11.29 2 12.21 22 11.45 3 2.31 22.20 11, 52 4 22.40 11.55 5 2.47 2260 11. 58 2.59 2296 11.60 7 2.61 23.00 11.63 8 2.69 23.20 1.65 9 2.17 23.40 11.66 lo 2.83 23.60 2.90 23.80 12 3.00 24.00 11.72 13 3.00 2.4.20 111.74 14 2.4.40 15 3.30 2.4.60 1.72 16 13.43 24.80 1.78 17. 25.00 11 se 18 5.82 25.20 7.81 19 14.32 25.90 11.82 NQ OH PH achledem 25.60 11. 84 25 80 25.00 11. 85 26.20 Z 26.40 26.00 89 26, 801 I. 90 27 2.7.20 1.92 27.40 1.93 27. 66 11.94 2.7.20 195 28 12-06 3.98 12.02 68 1.70 3.19 11.76 33 3.61 34 12.07 12.09 12.12 12.13 12.15 100 35 36 37 12.12 61 38 39 40 12.19 12 2.0 12 21 pH at half equavilant point =pka You are required to perform the following tasks before you leave the test: NaOH = 0.1 moldms 1. Plot a suitable pH titration graph. Hence determine (a) The concentration of the unknown acid (b) The acid dissociation constant -pka 2. Determine a value for the cell constant. ka-10 D F G G H K 14 12 . . . o . . lo 9 10 00 8 pH 6 4 . . O Series "pH" Point 19" (19.4.32) O . . O . . O O N 2 o 0 45 40 35 5 0 15 10 Volume NaOH added tm3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


