Question: 2. Hydrobromic acid (HBr) is a strong acid, while hypochlorous acid is a weak acid. Their respective dissociation reactions and acid dissociation constants are shown
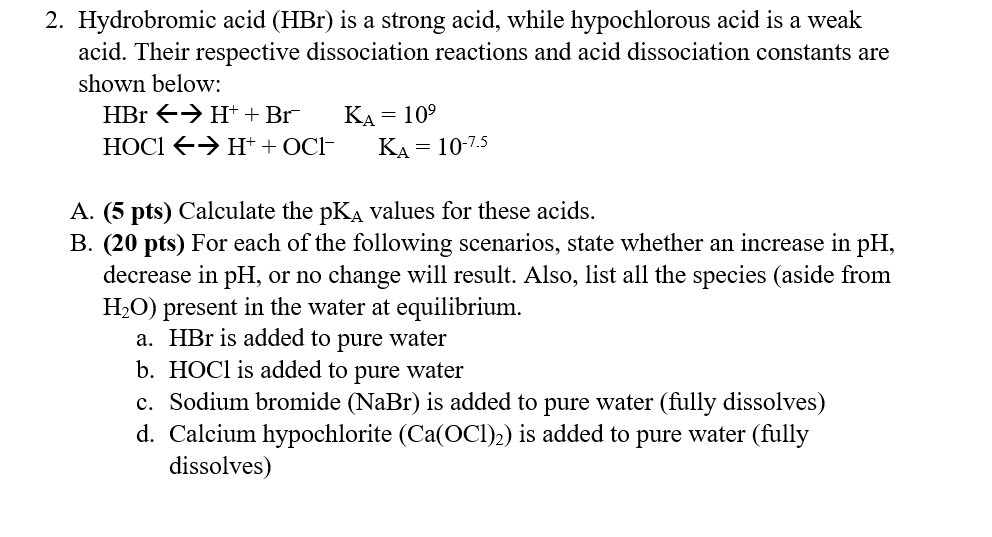
2. Hydrobromic acid (HBr) is a strong acid, while hypochlorous acid is a weak acid. Their respective dissociation reactions and acid dissociation constants are shown below: HBr > H+ + Br KA = 109 HOCIH+ + OCl- KA = = 10-7.5 A. (5 pts) Calculate the pKA values for these acids. B. (20 pts) For each of the following scenarios, state whether an increase in pH, decrease in pH, or no change will result. Also, list all the species (aside from H2O) present in the water at equilibrium. a. HBr is added to pure water b. HOCI is added to pure water c. Sodium bromide (NaBr) is added to pure water (fully dissolves) d. Calcium hypochlorite (Ca(OCT)2) is added to pure water (fully dissolves)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


