Question: 2. in a PER(plug flow reactor) under steady state, the following gas reaction takes place. The rate equation is given below. Where, k=3hr1 and K0=104(L/mol)2.
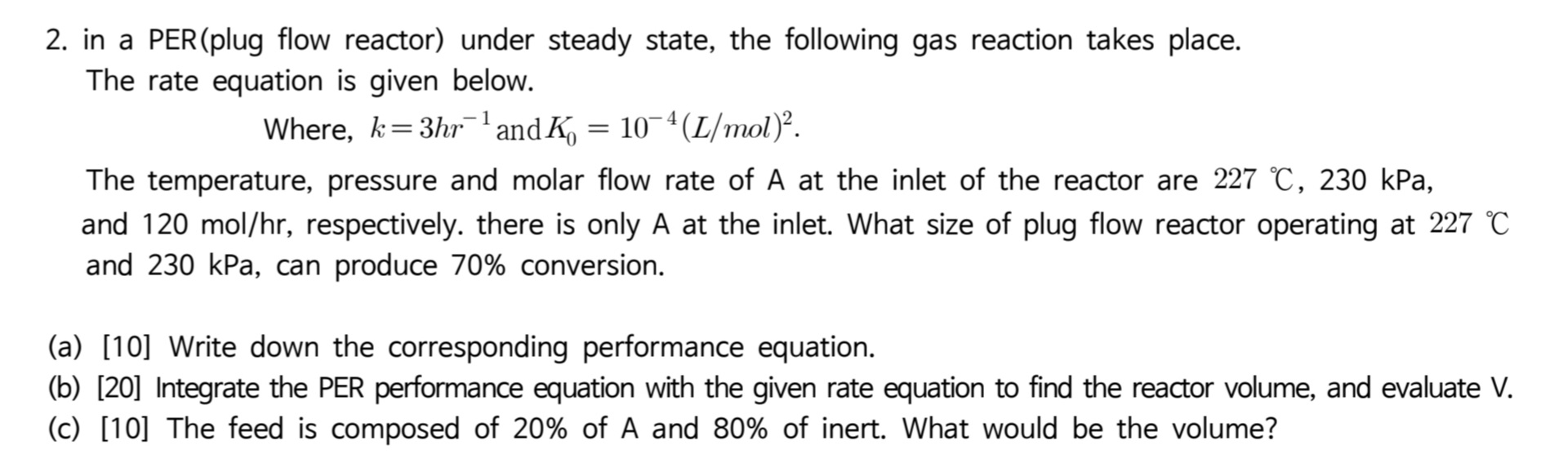
2. in a PER(plug flow reactor) under steady state, the following gas reaction takes place. The rate equation is given below. Where, k=3hr1 and K0=104(L/mol)2. The temperature, pressure and molar flow rate of A at the inlet of the reactor are 227C,230kPa, and 120mol/hr, respectively. there is only A at the inlet. What size of plug flow reactor operating at 227C and 230kPa, can produce 70% conversion. (a) [10] Write down the corresponding performance equation. (b) [20] Integrate the PER performance equation with the given rate equation to find the reactor volume, and evaluate V. (c) [10] The feed is composed of 20% of A and 80% of inert. What would be the volume
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


