Question: 2 questions Enter your answer in the provided box. How many grams of phosphine (PH3) can form when 18.7g of phosphorus and 93.1L of hydroge
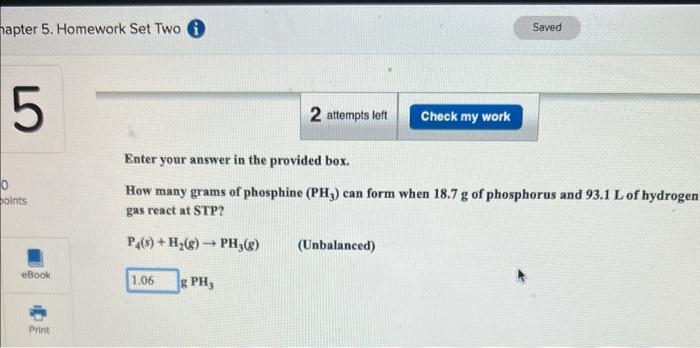
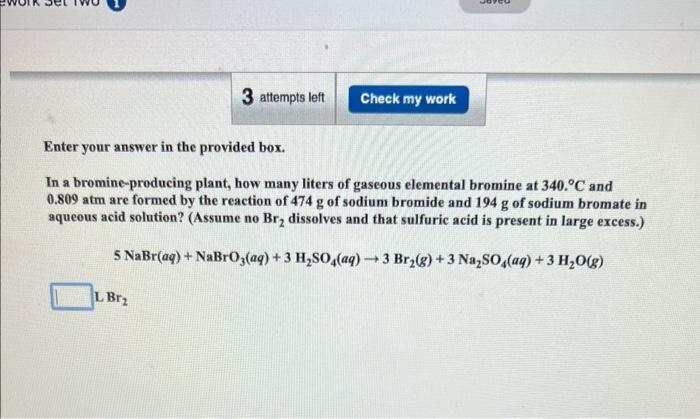
Enter your answer in the provided box. How many grams of phosphine (PH3) can form when 18.7g of phosphorus and 93.1L of hydroge gas react at STP? P4(s)+H2(g)PH3(g) (Unbalanced) Enter your answer in the provided box. In a bromine-producing plant, how many liters of gaseous elemental bromine at 3400C and 0.809atm are formed by the reaction of 474g of sodium bromide and 194g of sodium bromate in aqueous acid solution? (Assume no Br2 dissolves and that sulfuric acid is present in large excess.) 5NaBr(aq)+NaBrO3(aq)+3H2SO4(aq)3Br2(g)+3Na2SO4(aq)+3H2O(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


