Question: Enter your answer in the provided box. How many grams of phosphine (PH3) can form when 34.6g of phosphorus and 85.5L of hydrogen gas react
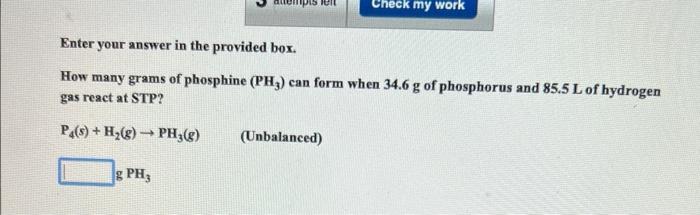
Enter your answer in the provided box. How many grams of phosphine (PH3) can form when 34.6g of phosphorus and 85.5L of hydrogen gas react at STP? P4(s)+H2(g)PH3(g) (Unbalanced) g PH3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


