Question: 2 S O 2 + O 2 = 2 S O 3 S O 3 + H 2 S O 4 = H 2 S
With the folliwing reactions and knowing we get kg of HSO at the end, determine the composition of all of the different components on this reactions.
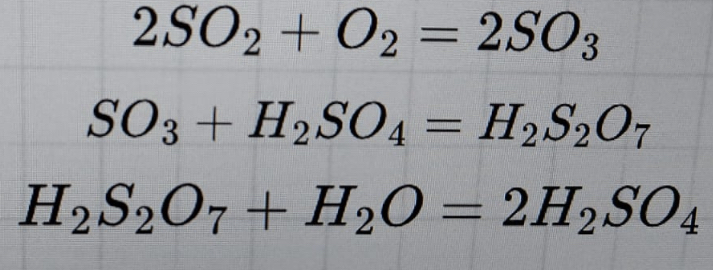
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


