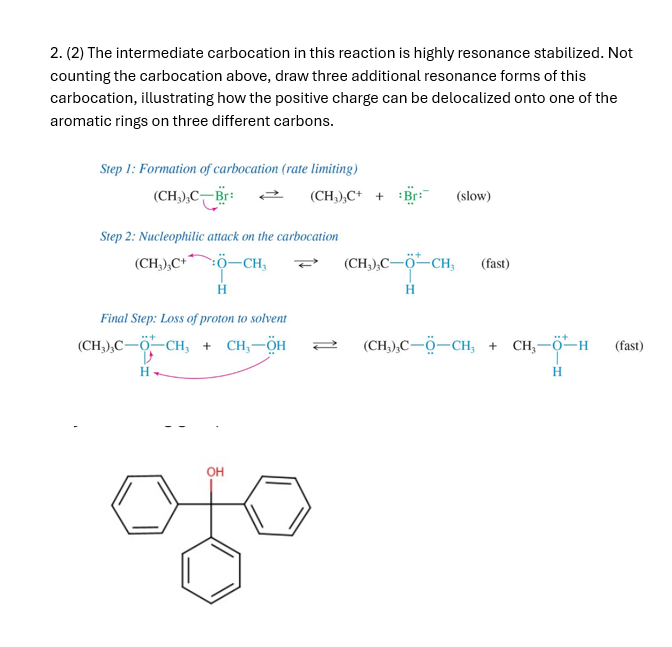
Question: ( 2 ) The intermediate carbocation in this reaction is highly resonance stabilized. Not counting the carbocation above, draw three additional resonance forms of this
The intermediate carbocation in this reaction is highly resonance stabilized. Not
counting the carbocation above, draw three additional resonance forms of this
carbocation, illustrating how the positive charge can be delocalized onto one of the
aromatic rings on three different carbons.
Step : Formation of carbocation rate limiting
Step : Nucleophilic attack on the carbocation
fast
Final Step: Loss of proton to solvent
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


