Question: help me with organic chem 10 ? SOLVED PROBLEM 11.16 Draw the major 1.2-addition and 1,4 addition products of this reaction Think How many distinct
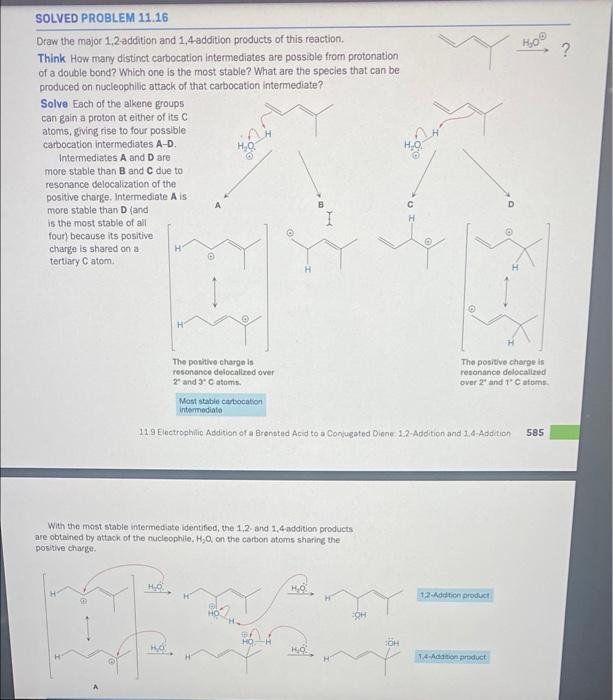
10 ? SOLVED PROBLEM 11.16 Draw the major 1.2-addition and 1,4 addition products of this reaction Think How many distinct carbocation intermediates are possible from protonation of a double bond? Which one is the most stable? What are the species that can be produced on nucleophilic attack of that carbocation intermediate? Solve Each of the alkene groups can gain a proton at either of its atoms, giving rise to four possible carbocation intermediates A-D. Intermediates A and D are more stable than B and C due to resonance delocalization of the positive charge. Intermediate Als more stable than D (and is the most stable of all four) because its positive charge is shared on a tertiary C atom 1 H H H The positive charge is resonance delocalized over 29 and 3 atoms Most stable carbocation Intermediate The positive charge is resonance delocalized over 2 and 1 atoms. 11.9 Electrophilic Addition of a Brenated Acid to a Conjugated Diane 1.2-Addition and 1.4-Addition 585 With the most stable intermediate identified, the 1.2 and 1.4-addition products are obtained by attack of the nucleophile. Hy on the carbon atoms sharing the positive charge HO 1.2 Addition product CH SOH HO HO 14 Action product
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


