Question: (2): The potential energy E per K-Cl pair within the KCl crystal depends on the interionic separation r in the same fashion as in the
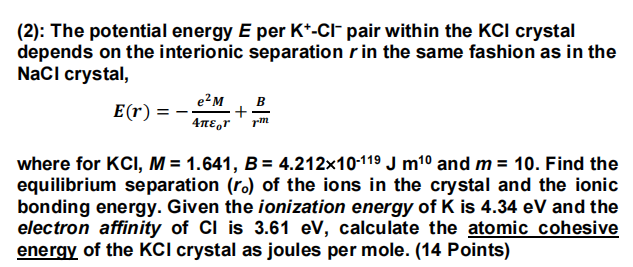
(2): The potential energy E per K-Cl pair within the KCl crystal depends on the interionic separation r in the same fashion as in the NaCl crystal, e-M E(r) = + 4,r B rm where for KCI, M = 1.641, B = 4.212x10-119 J m10 and m= 10. Find the equilibrium separation (ro) of the ions in the crystal and the ionic bonding energy. Given the ionization energy of K is 4.34 eV and the electron affinity of Cl is 3.61 eV, calculate the atomic cohesive energy of the KCI crystal as joules per mole. (14 Points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


