Question: Consider ionic bonding in CsCl (is it ionic?!) The potential energy E per Cs+Clpair within the CsCl crystal depends on the interionic separation r in
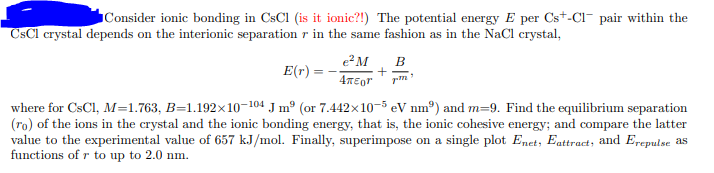
Consider ionic bonding in CsCl (is it ionic?!) The potential energy E per Cs+Clpair within the CsCl crystal depends on the interionic separation r in the same fashion as in the NaCl crystal, E(r)=40re2M+rmB, where for CsCl, M=1.763,B=1.19210104Jm9 (or 7.442105eVnm9 ) and m=9. Find the equilibrium separation (r0) of the ions in the crystal and the ionic bonding energy, that is, the ionic cohesive energy; and compare the latter value to the experimental value of 657kJ/mol. Finally, superimpose on a single plot Enet,Eattract, and Erepulse as functions of r to up to 2.0nm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


