Question: 2. The reaction was given below. a. Ca(OH)2 + b. H2SO4- - c. CaSO4 + d. H20 If you start with 18.82 gr of
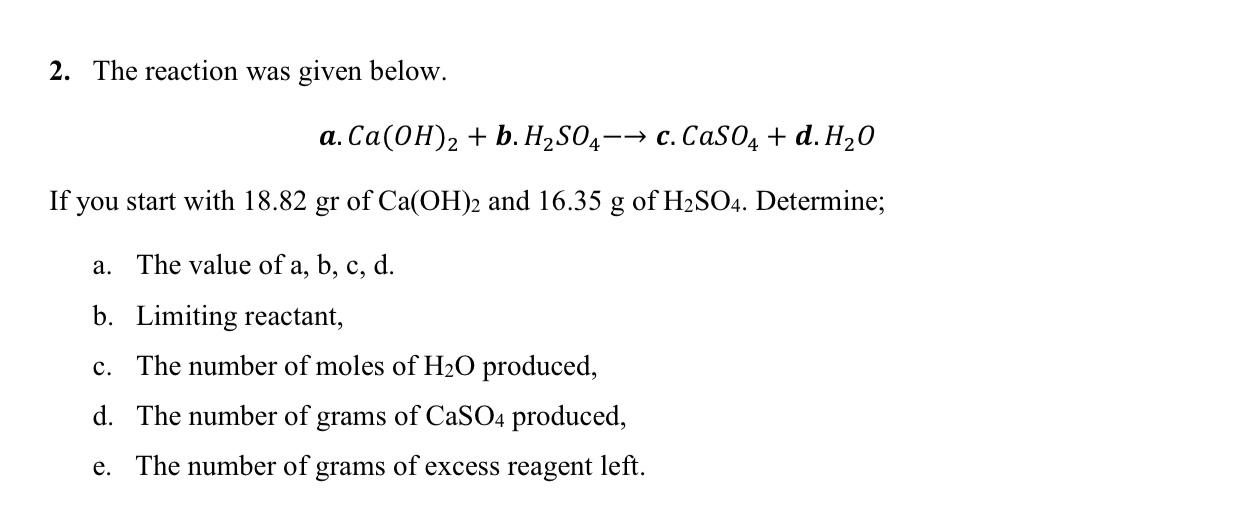
2. The reaction was given below. a. Ca(OH)2 + b. H2SO4- - c. CaSO4 + d. H20 If you start with 18.82 gr of Ca(OH)2 and 16.35 g of H2SO4. Determine; a. The value of a, b, c, d. b. Limiting reactant, c. The number of moles of H20 produced, d. The number of grams of CaSO4 produced, e. The number of grams of excess reagent left.
Step by Step Solution
3.39 Rating (165 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


