Question: 1. There are twenty common cations that can be analyzed readily in aqueous solution. These cations can be divided into five groups according to
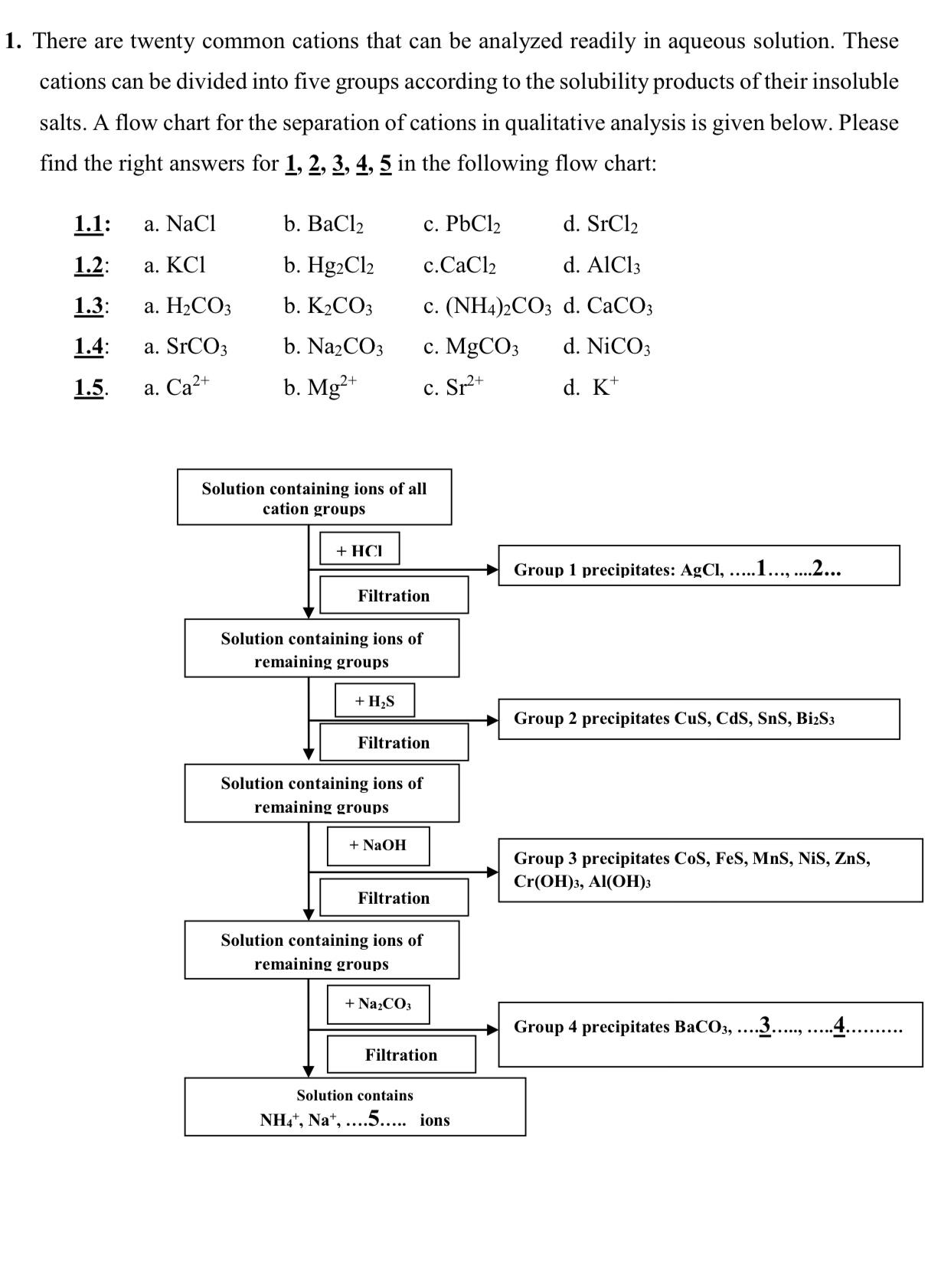
1. There are twenty common cations that can be analyzed readily in aqueous solution. These cations can be divided into five groups according to the solubility products of their insoluble salts. A flow chart for the separation of cations in qualitative analysis is given below. Please find the right answers for 1, 2, 3, 4, 5 in the following flow chart: 1.1: a. NaCl b. Clz c. PbCl2 d. SrCl2 1.2: a. KCI b. Hg2Cl2 c.CaCl2 d. AlCl3 1.3: . 2 b. K2CO3 c. (NH4)2CO3 d. CaCO3 1.4: a. SrCO3 b. NazCO3 . MgCO d. NICO3 1.5. . 2+ b. Mg* c. Sr2+ d. K* Solution containing ions of all cation groups + HCI Group 1 precipitates: AgCl, ...1.., .2... Filtration Solution containing ions of remaining groups + H,S Group 2 precipitates CuS, CdS, SnS, BizS3 Filtration Solution containing ions of remaining groups + NaOH Group 3 precipitates CoS, FeS, MnS, NiS, ZnS, Cr(H), AlI(O) Filtration Solution containing ions of remaining groups + Na2CO3 Group 4 precipitates BaCO3, ..3... Filtration Solution contains NH,", Na*, ..5... ions
Step by Step Solution
3.38 Rating (157 Votes )
There are 3 Steps involved in it
Group 1 precipitates are PbCl2 AgCl and ... View full answer

Get step-by-step solutions from verified subject matter experts


