Question: 2) The relationship between mean free path, collision frequency, and mean (average) speed for a pure gas is z=vmen=(8RT/M)12 Therefore, if we can find an
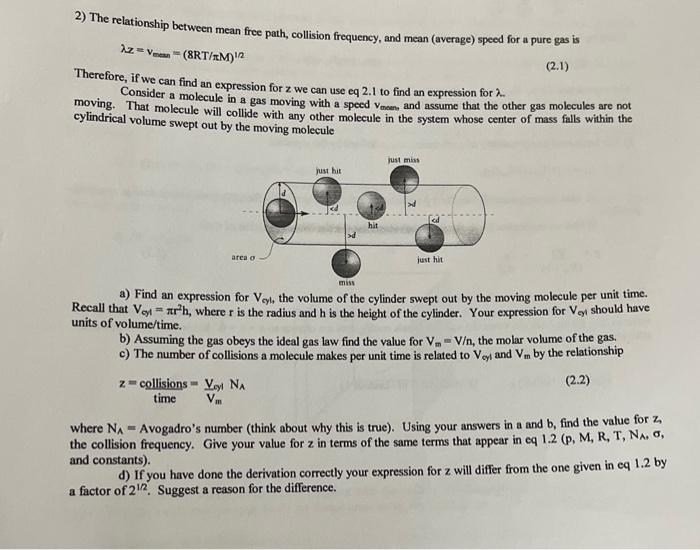
2) The relationship between mean free path, collision frequency, and mean (average) speed for a pure gas is z=vmen=(8RT/M)12 Therefore, if we can find an expression for z we can use eq 2.1 to find an expression for . Consider a molecule in a gas moving with a speed vmann and assume that the other gas molecules are not moving. That molecule will collide with any other molecule in the system whose center of mass falls within the cylindrical volume swept out by the moving molecule a) Find an expression for Vcyl, the volume of the cylinder swept out by the moving molecule per unit time. Recall that Voyl=r2h, where r is the radius and h is the height of the cylinder. Your expression for Vel should have units of volume/time. b) Assuming the gas obeys the ideal gas law find the value for Vm=V, the molar volume of the gas. c) The number of collisions a molecule makes per unit time is related to Voy and Vm by the relationship z=timecollisions=VmVollNA where NA= Avogadro's number (think about why this is true). Using your answers in a and b,findthevaluefor, the collision frequency. Give your value for z in terms of the same terms that appear in eq 1.2(p,M,R,T,N, , and constants). d) If you have done the derivation correctly your expression for z will differ from the one given in eq 1.2 by a factor of 21/2. Suggest a reason for the difference
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


