Question: 2. Using a dashed line to represent a hydrogen bond, draw Lewis structures (i.e., show all valence electrons and formal charges) showing two ways each
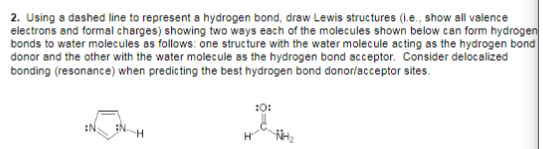
2. Using a dashed line to represent a hydrogen bond, draw Lewis structures (i.e., show all valence electrons and formal charges) showing two ways each of the molecules shown below can form hydrogen bonds to water molecules as follows: one structure with the water molecule acting as the hydrogen bond donor and the other with the water molecule as the hydrogen bond acceptor. Consider delocalized bonding (resonance) when predicting the best hydrogen bond donor/acceptor sites. :0: :N H
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


