Question: 2. Using the data provided in the table above, determine the rate law for this reaction. 3. Calculate the rate constant for each solution. Include
2. Using the data provided in the table above, determine the rate law for this reaction.
3. Calculate the rate constant for each solution. Include the units of the rate constant for each solution in your answer.
Solution 1: Solution 2: Solution 3:
4. Do you expect the rate constant for each solution to be the same or different? Explain the reasoning behind your answer.
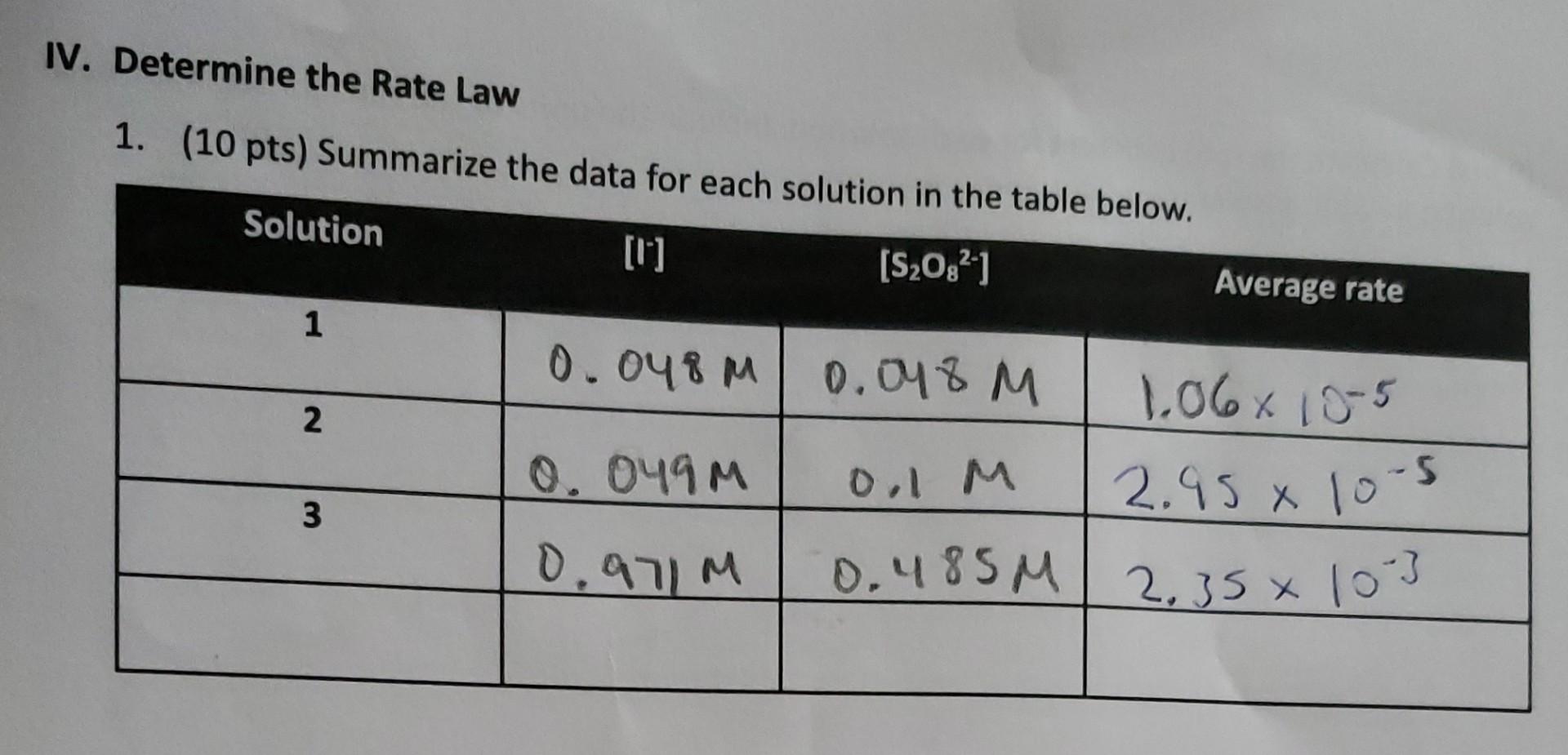
IV. Determine the Rate Law 1. (10 pts) Summarize the data for each solution in the table below. Solution [S2082-) Average rate 1 0.048m 0.048 M % 1.06x 10-5 2 0.049M 0,1 M 2.95 x 10-5 X 3 0.971M 0.485M 2,35% 103
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


