Question: Using data from the table below, determine the rate law for the following reaction. Then, calculate the value and units of the rate constant, k.
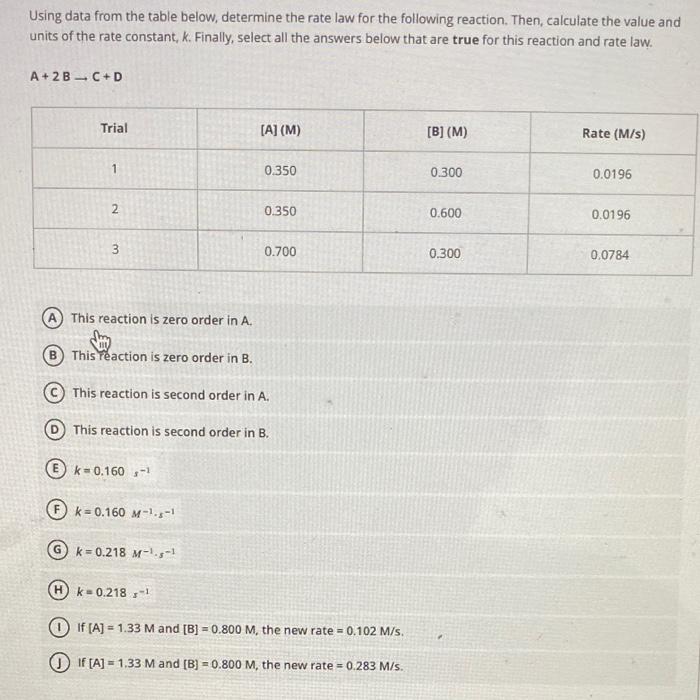
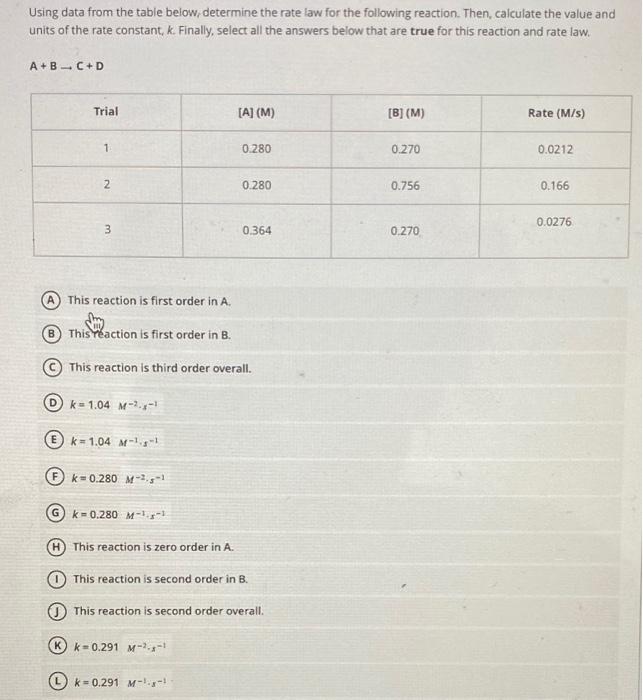
Using data from the table below, determine the rate law for the following reaction. Then, calculate the value and units of the rate constant, k. Finally, select all the answers below that are true for this reaction and rate law. A+2BC+D (A) This reaction is zero order in A. (B) This reaction is zero order in B. (C) This reaction is second order in A. (D) This reaction is second order in B. (E) k=0.160s1 (F) k=0.160M1s1 (G) k=0.218M1s1 (H) k=0.218x1 (1) If [A]=1.33M and [B]=0.800M, the new rate =0.102M/s. (1) If [A]=1.33M and [B]=0.800M, the new rate =0.283M/s. Using data from the table below; determine the rate law for the following reaction. Then, calculate the value and units of the rate constant, k. Finally, select all the answers below that are true for this reaction and rate law. A+BC+D (A) This reaction is first order in A. (B) This teaction is first order in B. (C) This reaction is third order overall. (D) k=1.04M2s1 (E) k=1.04M1s1 (F) k=0.280M2s1 (G) k=0.280M1s1 (H) This reaction is zero order in A. (1) This reaction is second order in B. (J) This reaction is second order overall. (K) k=0.291M2x1 (L) k=0.291M1x1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


