Question: 2) Which carbocation would be least stable? 3) Which carbocation would be most stable? a) b) + CH2CH2CH=CHCH3 c) d) e) 4) Which of the
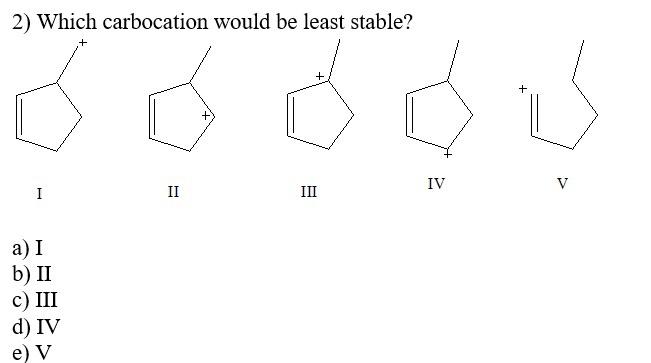
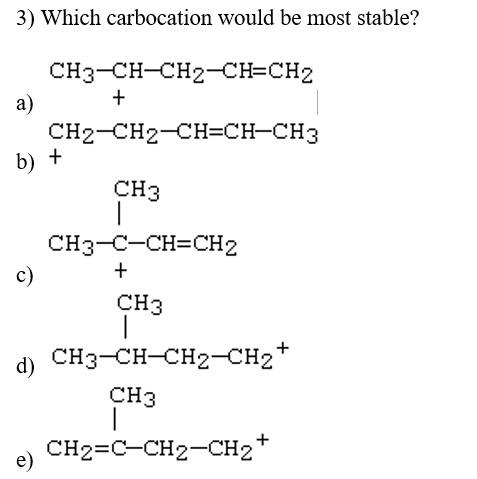

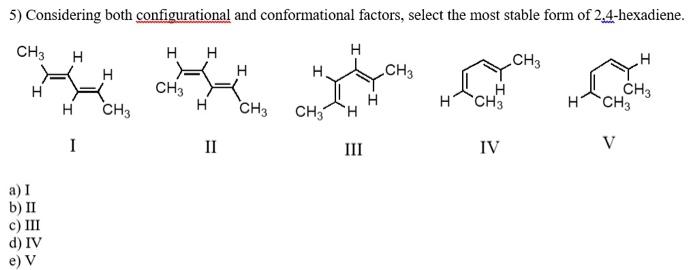
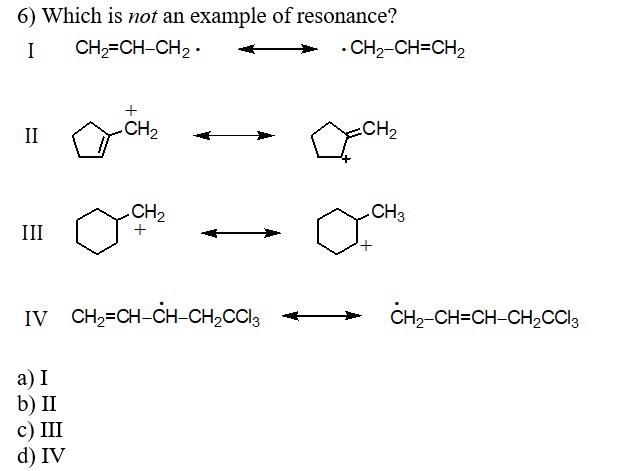
2) Which carbocation would be least stable? 3) Which carbocation would be most stable? a) b) + CH2CH2CH=CHCH3 c) d) e) 4) Which of the following dienes would you expect to be the most stable? a) CH3CH=CHCH=CHCH3 b) CH3CH=CHCH2CH=CH2 c) CH2=CHCH2CH2CH=CH2 d) CH2=CHCH(CH3)CH=CH2 5) Considering both configurational and conformational factors, select the most stable form of 2,4 -hexadiene. I I III IV V 6) Which is not an example of resonance? I CH2=CHCH2CH2CH=CH2 II III IV CH2=CHCHCH2CCl3CH2CH=CHCH2CCl3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


