Question: (20 points) Consider a binary liquid mixture of 20 mole % ethanol (1) and water (2) solution at 78C and 1 atm. The activity coefficients
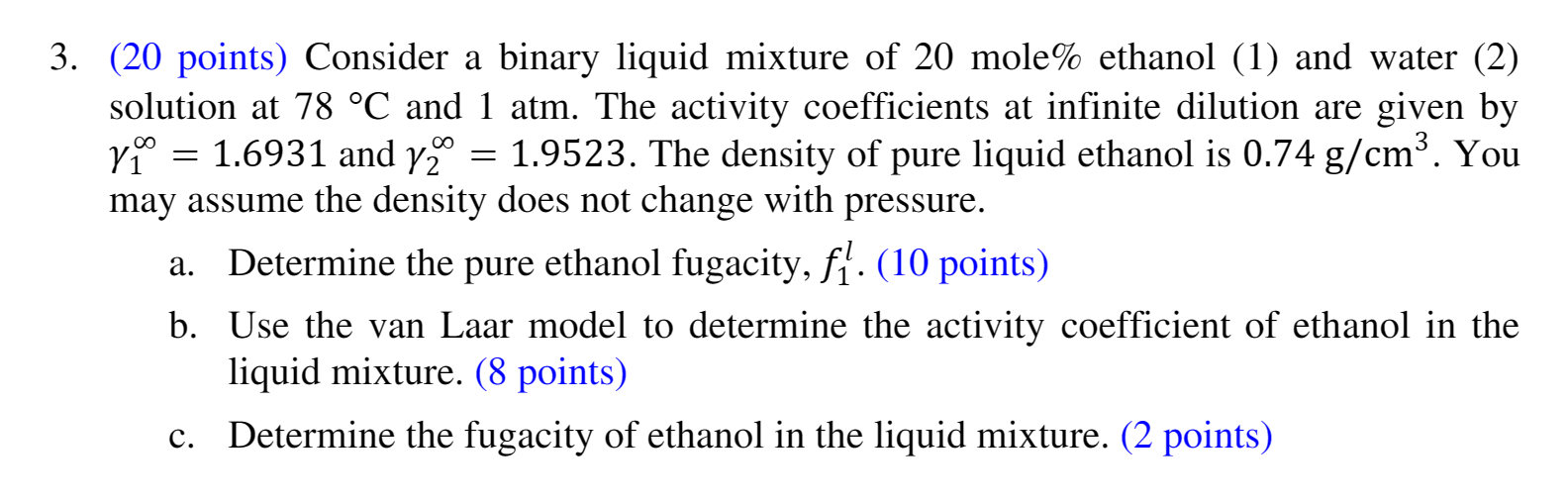
(20 points) Consider a binary liquid mixture of 20 mole % ethanol (1) and water (2) solution at 78C and 1 atm. The activity coefficients at infinite dilution are given by may assume the density does not change with pressure. a. Determine the pure ethanol fugacity, f1l. (10 points) b. Use the van Laar model to determine the activity coefficient of ethanol in the liquid mixture. (8 points) c. Determine the fugacity of ethanol in the liquid mixture. (2 points)
Step by Step Solution
There are 3 Steps involved in it
To solve the question we will break down each part systematically Given Data Binary liquid mixture 20 mole ethanol 1 and water 2 Temperature 78C Press... View full answer

Get step-by-step solutions from verified subject matter experts


