Question: 20-16. In this problem, we will illustrate the condition dSprod0 with a concrete example. Consider the two-component system shown in Figure 20.8. Each compartment is
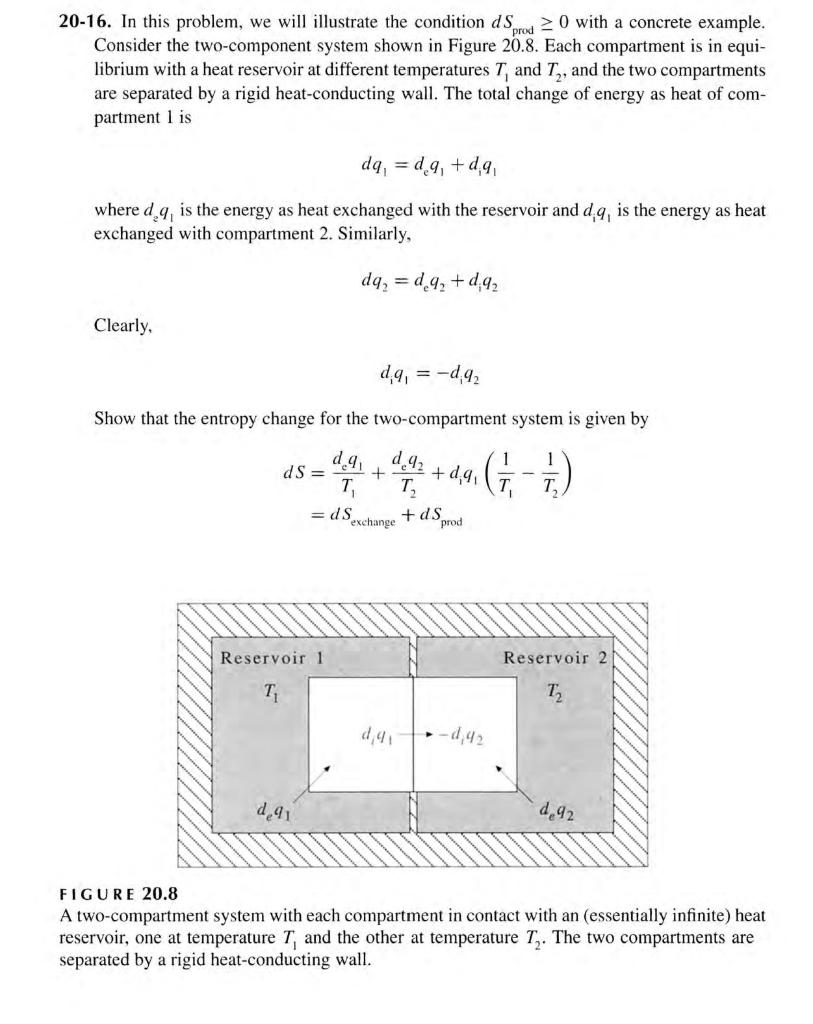
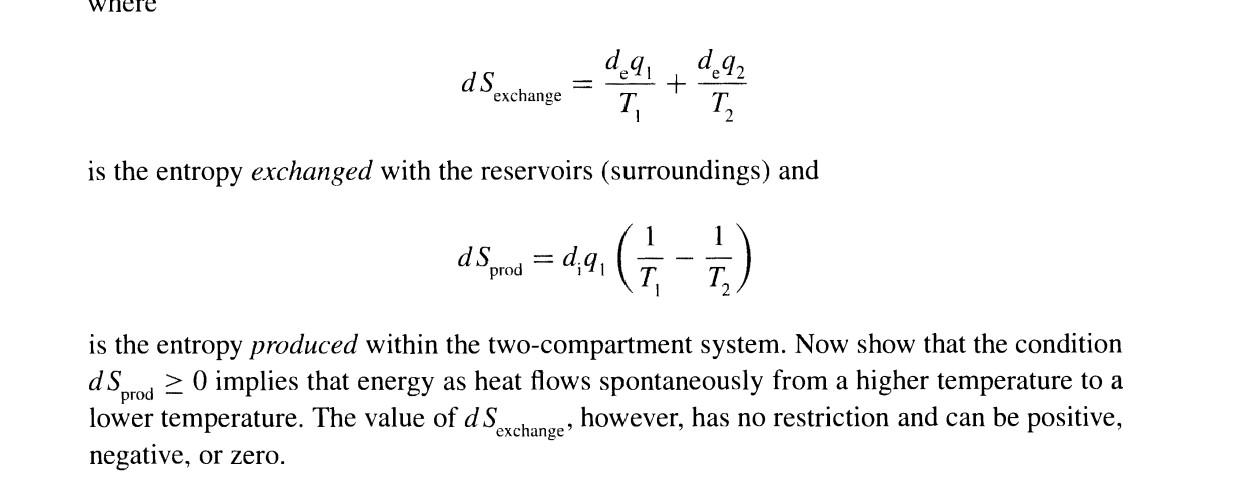
20-16. In this problem, we will illustrate the condition dSprod0 with a concrete example. Consider the two-component system shown in Figure 20.8. Each compartment is in equilibrium with a heat reservoir at different temperatures T1 and T2, and the two compartments are separated by a rigid heat-conducting wall. The total change of energy as heat of compartment 1 is dq1=deq1+d1q1 where d2q1 is the energy as heat exchanged with the reservoir and d1q1 is the energy as heat exchanged with compartment 2 . Similarly, dq2=deq2+diq2 Clearly, diq1=diq2 Show that the entropy change for the two-compartment system is given by dS=T1deq1+T2deq2+diq1(T11T21)=dSexchange+dSprod F I GURE 20.8 A two-compartment system with each compartment in contact with an (essentially infinite) heat reservoir, one at temperature T1 and the other at temperature T2. The two compartments are separated by a rigid heat-conducting wall. dSexchange=T1deq1+T2deq2 is the entropy exchanged with the reservoirs (surroundings) and dSprod=diq1(T11T21) is the entropy produced within the two-compartment system. Now show that the condition dSprod0 implies that energy as heat flows spontaneously from a higher temperature to a lower temperature. The value of dSexchange, however, has no restriction and can be positive, negative, or zero
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


