Question: 21. Solid MO2 is reduced by dry H2 gas to solid M at 1200C and 1 atm pressure in accord with the reaction: MO2(s) +
21. Solid MO2 is reduced by dry H2 gas to solid M at 1200C and 1 atm pressure in accord with the reaction: MO2(s) + 2H2(g) = M(s) + 2H2O(g) a) What is the PH2/PH2O ratio of the out coming gas if equilibrium is attained in the furnace? b) How many moles of MO2 is reduced by 1 mole of dry H2 gas entering into the furnace if equilibrium is attained in the furnace?
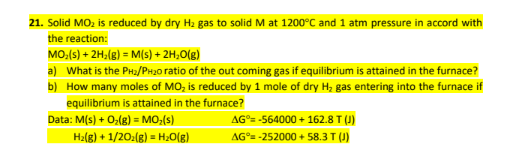
1. Solid MO2 is reduced by dry H2 gas to solid M at 1200C and 1 atm pressure in accord with the reaction: MO2(s)+2H2(g)=M(s)+2H2O(g) a) What is the PH2/PH2O ratio of the out coming gas if equilibrium is attained in the furnace? b) How many moles of MO2 is reduced by 1 mole of dry H2 gas entering into the furnace if equilibrium is attained in the furnace? Data:M(s)+O2(g)=MO2(s)H2(g)+1/2O2(g)=H2O(g)G=564000+162.8T(J)G=252000+58.3T(J)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


