Question: 23 7.6 From experimental data it is known that at moderate pressures the volumetric equation of state may be written as PV = RT +
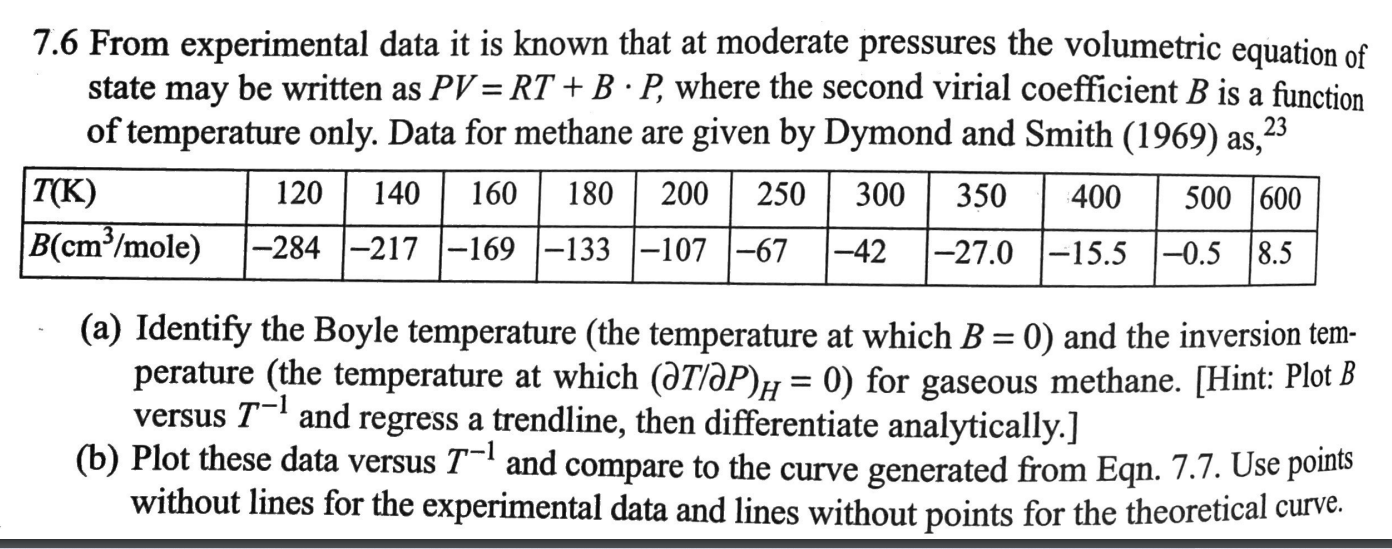
23 7.6 From experimental data it is known that at moderate pressures the volumetric equation of state may be written as PV = RT + B P, where the second virial coefficient B is a function of temperature only. Data for methane are given by Dymond and Smith (1969) as, T(K) 120 140 160 200 250 300 350 400 500 1600 B(cm/mole) -284 -217 -169 -133 -107 -67 -42 1-27.0 -15.5 -0.5 8.5 60 | 180 = (a) Identify the Boyle temperature (the temperature at which B = 0) and the inversion tem- perature (the temperature at which (atlP)h = 0) for gaseous methane. (Hint: Plot B versus T-1 and regress a trendline, then differentiate analytically.] (b) Plot these data versus T-1 and compare to the curve generated from Eqn. 7.7. Use points without lines for the experimental data and lines without points for the theoretical curve
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


