Question: Question Hexa-methyl-di-siloxane (HMDSO) is used as a raw material for the gas-phase synthesis of silicon dioxide (SiO2). The liquid HMDSO is heated to 70 C
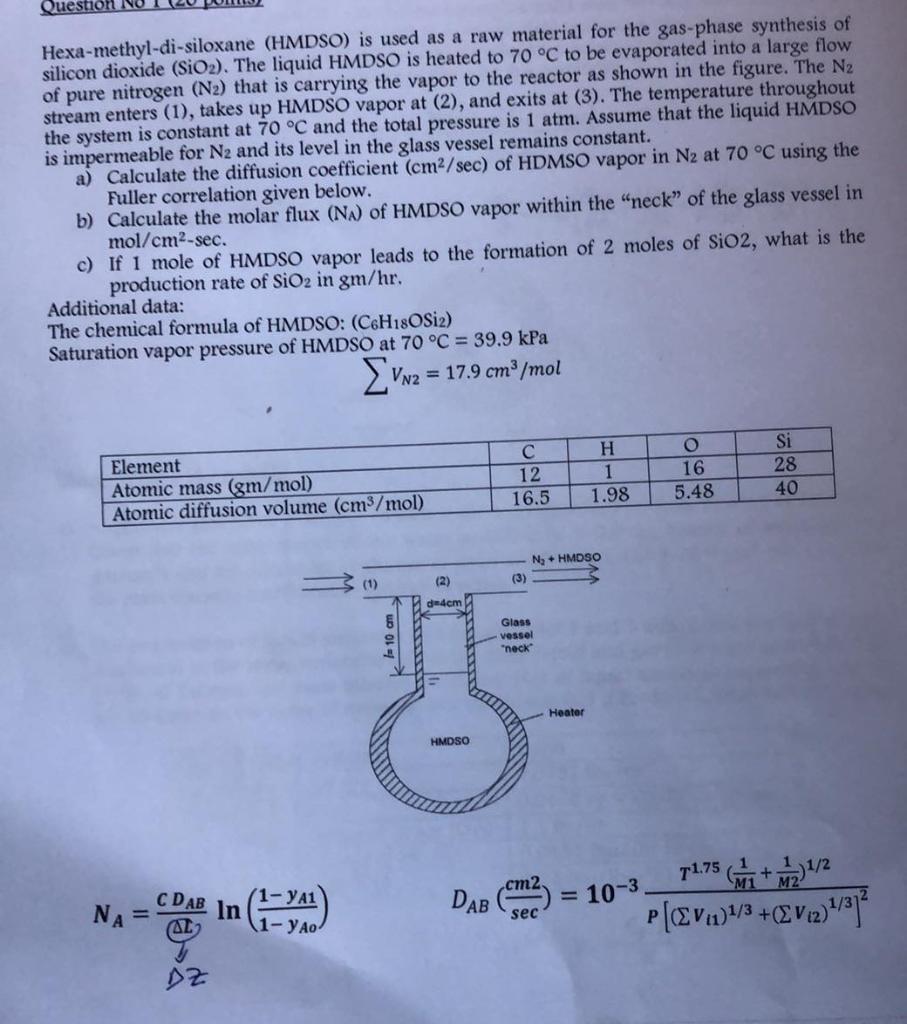
Question Hexa-methyl-di-siloxane (HMDSO) is used as a raw material for the gas-phase synthesis of silicon dioxide (SiO2). The liquid HMDSO is heated to 70 C to be evaporated into a large flow of pure nitrogen (N2) that is carrying the vapor to the reactor as shown in the figure. The N2 stream enters (1), takes up HMDSO vapor at (2), and exits at (3). The temperature throughout the system is constant at 70 C and the total pressure is 1 atm. Assume that the liquid HMDSO is impermeable for N2 and its level in the glass vessel remains constant. a) Calculate the diffusion coefficient (cm2/sec) of HDMSO vapor in N2 at 70 C using the Fuller correlation given below. b) Calculate the molar flux (N.) of HMDSO vapor within the "neck" of the glass vessel in mol/cm2-sec. c) If i mole of HMDSO vapor leads to the formation of 2 moles of SiO2, what is the production rate of SiO2 in gm/hr. Additional data: The chemical formula of HMDSO: (C6H18OSiz) Saturation vapor pressure of HMDSO at 70 C = 39.9 kPa vv2 = 17.9 cm /mol Element Atomic mass (gm/mol) Atomic diffusion volume (cm3/mol) 12 16.5 H 1 1.98 O 16 5.48 Si 28 40 Ny HMDSO (2) (1) (3) dedem Glass vessel "neck Heater HMDSO 11.75 1.75 +2,112 M1 CDAB in ( NA = = 10-3 1 1-YAO DAB (m2) : sec p[@Von]/3+EV2) AL DZ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


