Question: 2-3. For the electron motion in the three dimensional cubic box, we assume that 14 electrons are placed into the allowed energy level of a
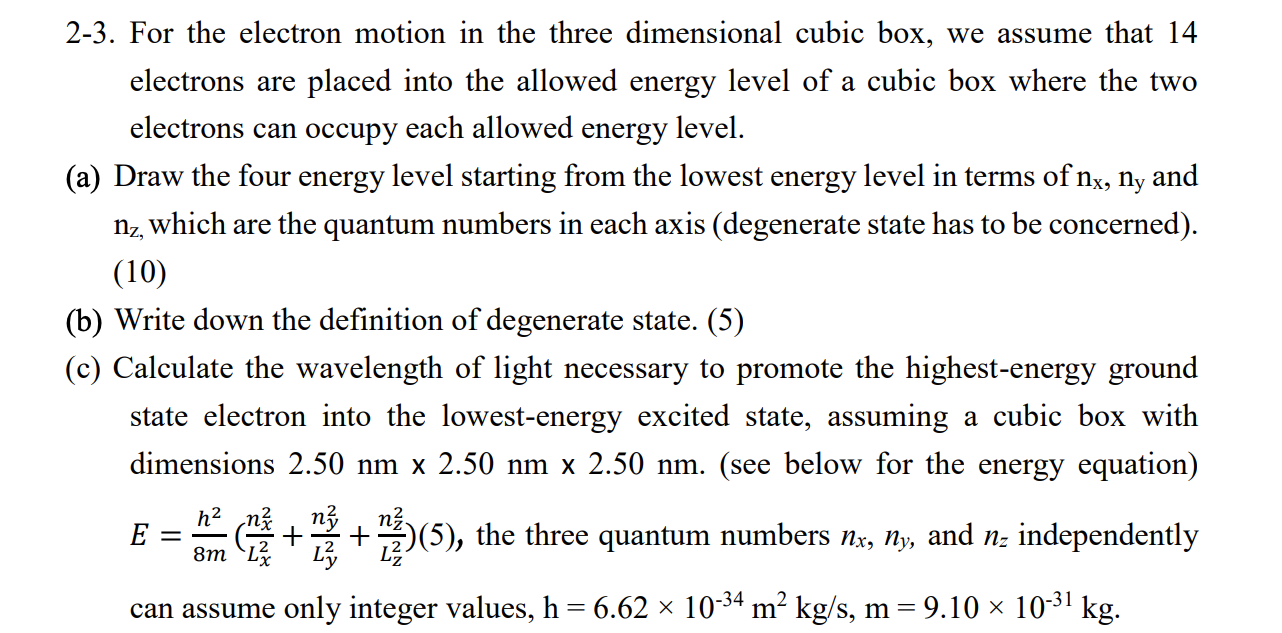
2-3. For the electron motion in the three dimensional cubic box, we assume that 14 electrons are placed into the allowed energy level of a cubic box where the two electrons can occupy each allowed energy level. (a) Draw the four energy level starting from the lowest energy level in terms of nx, ny and nz, which are the quantum numbers in each axis (degenerate state has to be concerned). (10) (b) Write down the definition of degenerate state. (5) (c) Calculate the wavelength of light necessary to promote the highest-energy ground state electron into the lowest-energy excited state, assuming a cubic box with dime sions 2.50 nm x 2.50 nm x 2.50 nm. (see below for the energy equation) E = E NA CEST (5), the three quantum numbers Ne, ny, and n: independently + can assume only integer values, h = 6.62 x 10-34 m kg/s, m = 9.10 ~ 10-31 kg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


