Question: + Half-life for First and Second Order Reactions 1 The half-life of a reaction, t1/2, is the time it takes for the reactant concentration [A]
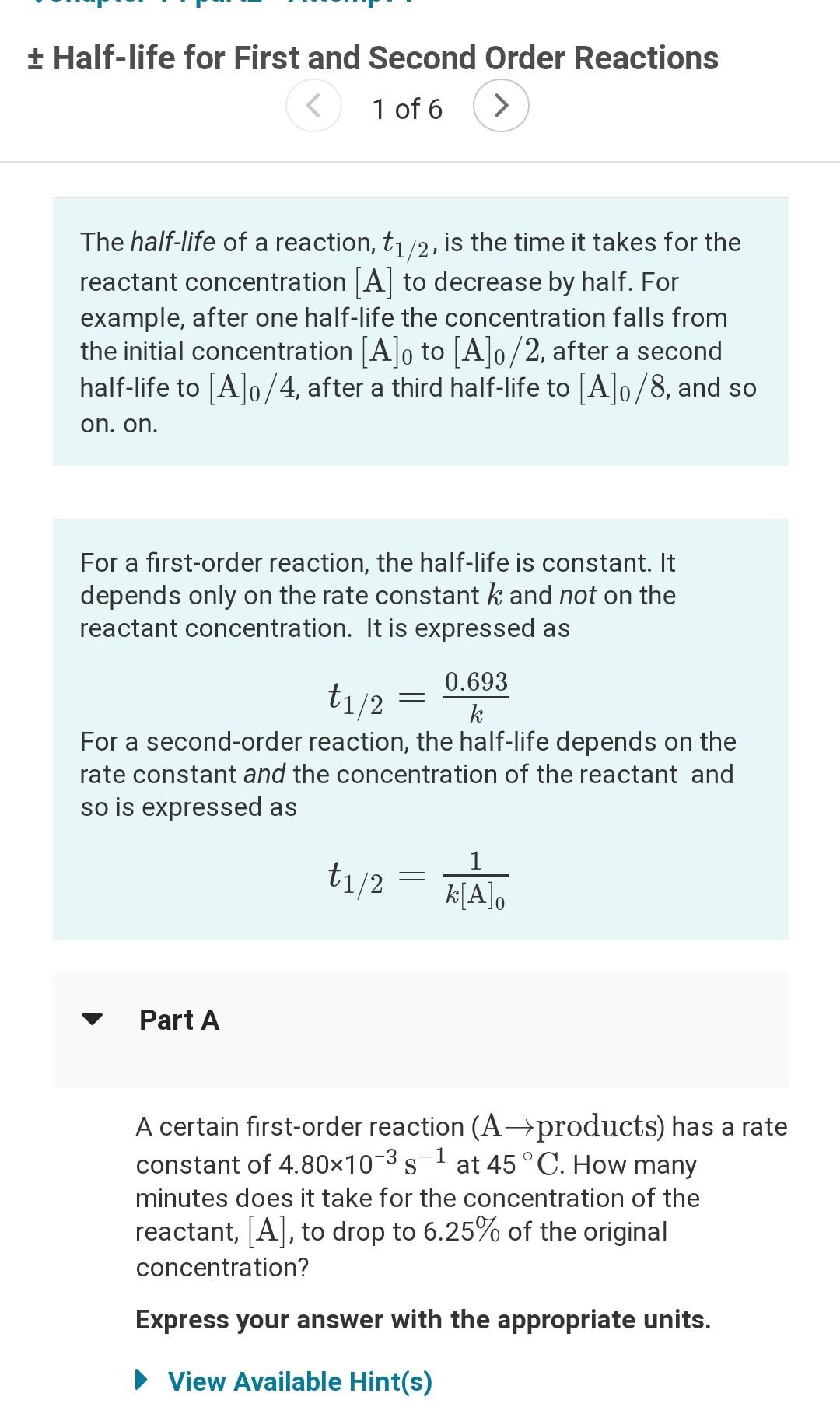
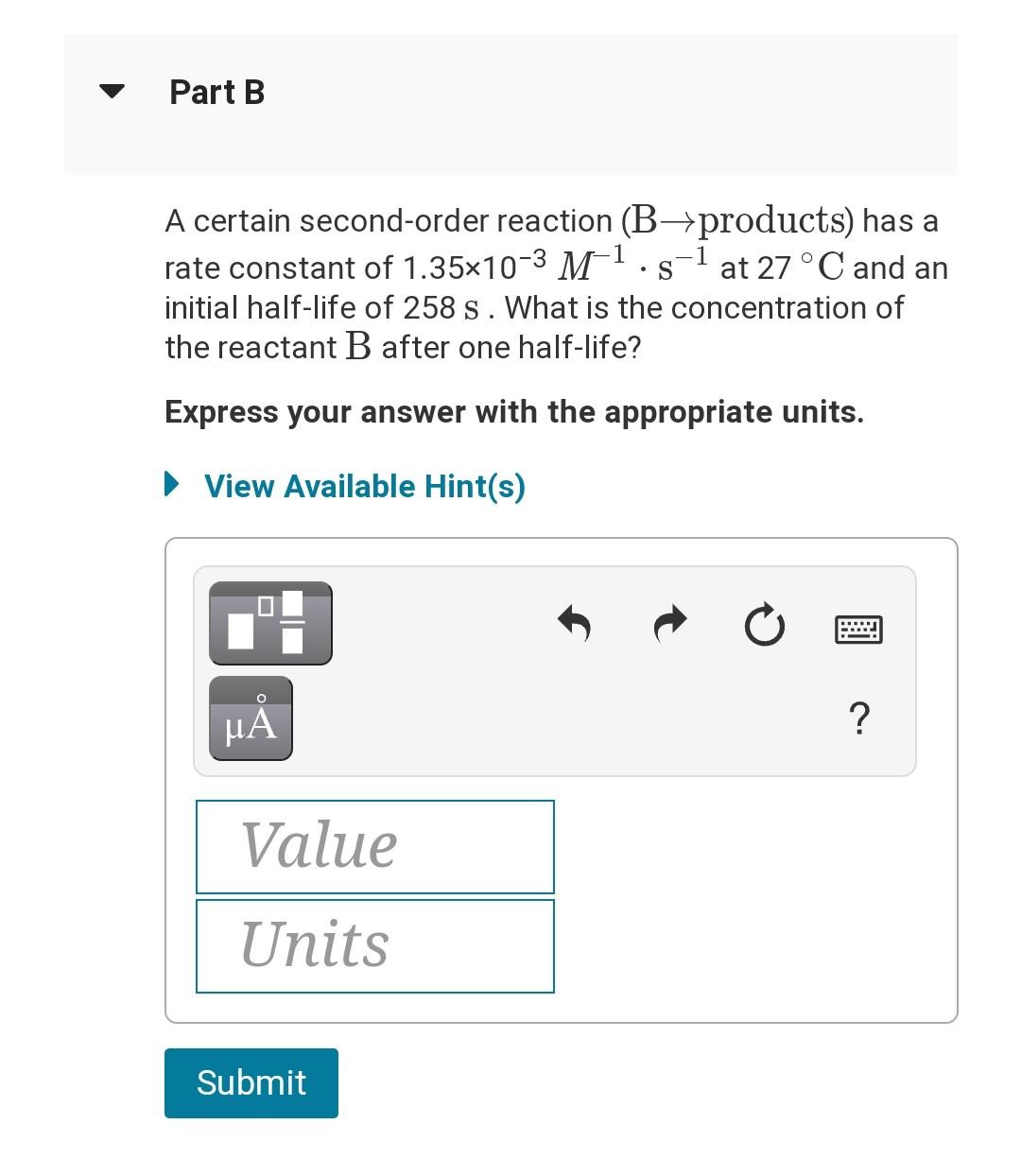
+ Half-life for First and Second Order Reactions 1 The half-life of a reaction, t1/2, is the time it takes for the reactant concentration [A] to decrease by half. For example, after one half-life the concentration falls from the initial concentration [A]o to [A]o/2, after a second half-life to [A]o/4, after a third half-life to [A]o/8, and so on. on. For a first-order reaction, the half-life is constant. It depends only on the rate constant k and not on the reactant concentration. It is expressed as - 0.693 t1/2 k For a second-order reaction, the half-life depends on the rate constant and the concentration of the reactant and so is expressed as t1/2 1 kA Part A -1 A certain first-order reaction (A+products) has a rate constant of 4.80x10-35 at 45 C. How many minutes does it take for the concentration of the reactant, [A], to drop to 6.25% of the original concentration? Express your answer with the appropriate units. View Available Hint(s) Part B A certain second-order reaction (B-products) has a rate constant of 1.35x10-3 M-1.s-1 at 27 C and an initial half-life of 258 s. What is the concentration of the reactant B after one half-life? Express your answer with the appropriate units. View Available Hint(s) ? Value Units Submit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


