Question: 23 Question 23 (10 points) 2 H2(g) + O2(g) 2 H2O(g) Use the following conversions: 1 mol MW in g = 22.414 L at STP
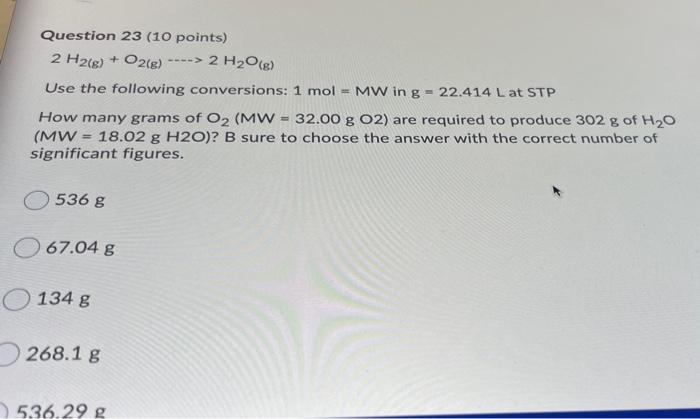
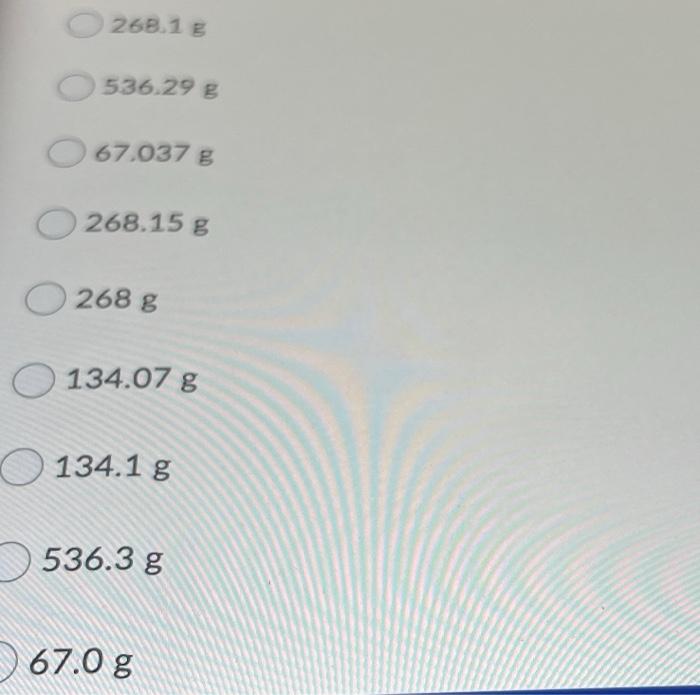
Question 23 (10 points) 2 H2(g) + O2(g) 2 H2O(g) Use the following conversions: 1 mol MW in g = 22.414 L at STP How many grams of O2 (MW = 32.00 g 02) are required to produce 302 g of H20 8 (MW = 18.02 g H20)? B sure to choose the answer with the correct number of significant figures. 5368 67.04 g O134 g 268.1 g 536.29 g 268.13 536.29 g 67.037 g 268.15 g 268 g 134.07 g 134.1 g 536.3 g 67.0 g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


