Question: 24 Given the rxn: 2NO+O2--->2N02 By what factor will the reaction rate change if the concentrations ?of both reactants are doubled 2) Rate = k[NO]?[02]
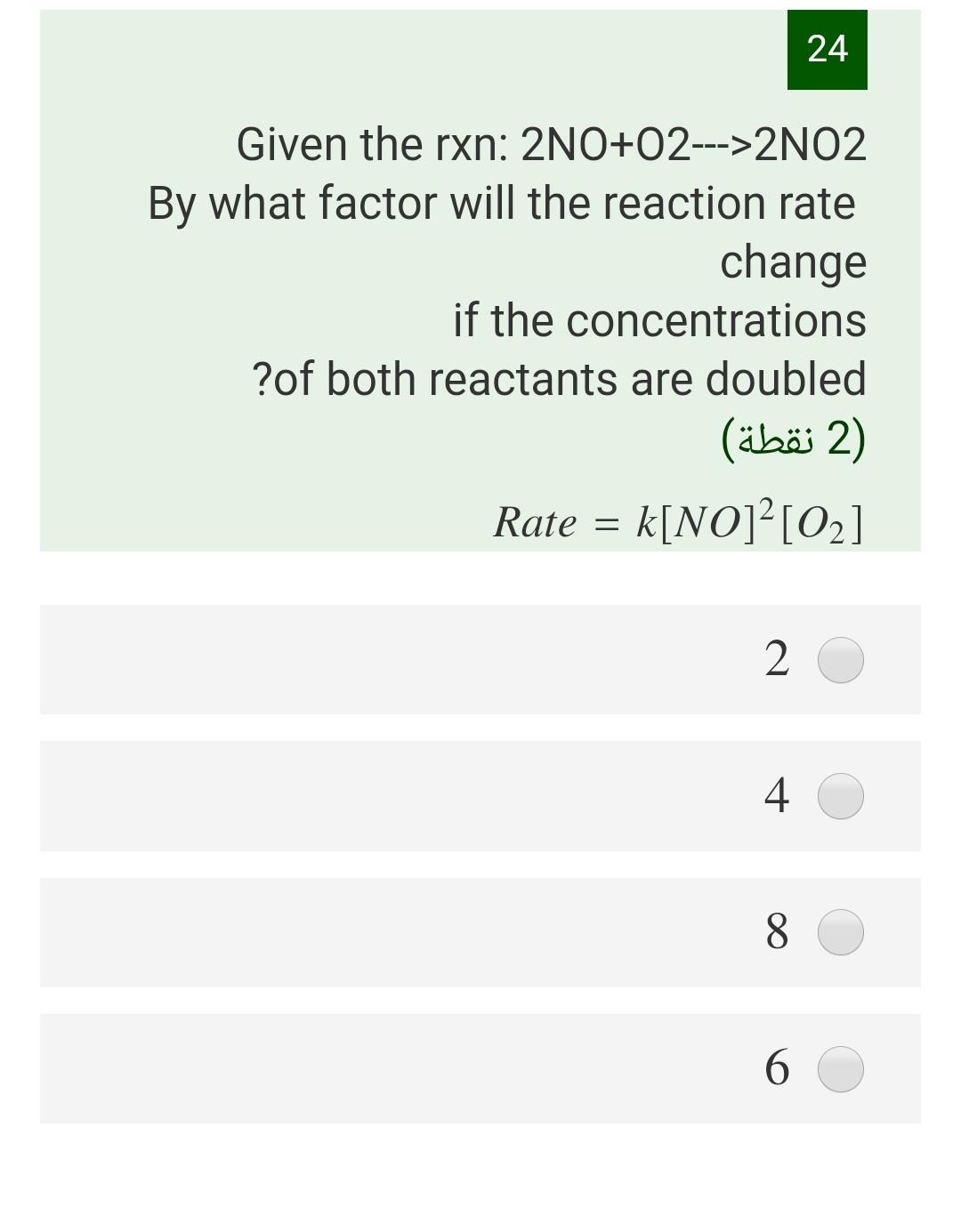
24 Given the rxn: 2NO+O2--->2N02 By what factor will the reaction rate change if the concentrations ?of both reactants are doubled 2) Rate = k[NO]?[02] (2 ) 2 4 8 6
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


