Question: 24.10 A gas separation process has been proposed to remove selectively two pollutants, hydrogen sulfide (H2S) and sulfur dioxide (SO2), from an exhaust gas stream
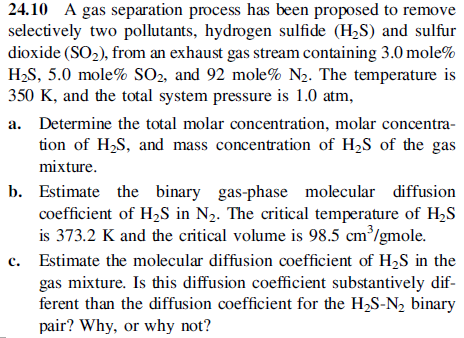
24.10 A gas separation process has been proposed to remove selectively two pollutants, hydrogen sulfide (H2S) and sulfur dioxide (SO2), from an exhaust gas stream containing 3.0 mole % H2S,5.0 mole %SO2, and 92 mole %N2. The temperature is 350K, and the total system pressure is 1.0atm, a. Determine the total molar concentration, molar concentration of H2S, and mass concentration of H2S of the gas mixture. b. Estimate the binary gas-phase molecular diffusion coefficient of H2S in N2. The critical temperature of H2S is 373.2K and the critical volume is 98.5cm3/ gmole. c. Estimate the molecular diffusion coefficient of H2S in the gas mixture. Is this diffusion coefficient substantively different than the diffusion coefficient for the H2SN2 binary pair? Why, or why not
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


