Question: (25 mins) Problem C: You must show work for part A, B and C on the written form to receive full credits. Consider the solution
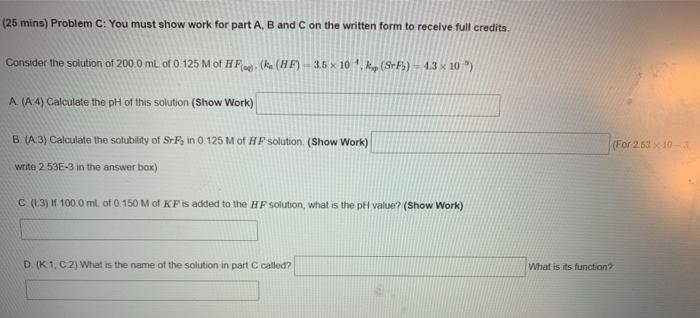
(25 mins) Problem C: You must show work for part A, B and C on the written form to receive full credits. Consider the solution of 200.0 mL of 0.125 M of HF. (R. (HF) - 3.5 x 10 kp (Srb) 4.3 x 20) A. (A 4) Calculate the pH of this solution (Show Work) (For 2.63 x 10 B. (:3) Calculate the solubility of S-Fin 0.125 M of HF solution. (Show Work) write 2 53E-3 in the answer box) C (13) 100.0 ml of 0.150 M of KF is added to the HF solution, what is the pH value? (Show Work) DK1, C2) What is the name of the solution in part C called? What is its function
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


