Question: 27 A solution is prepared which is acetic acid CH3COOH. What would happen to the pH of this solution if concentrated acetic acid CH3COOH was
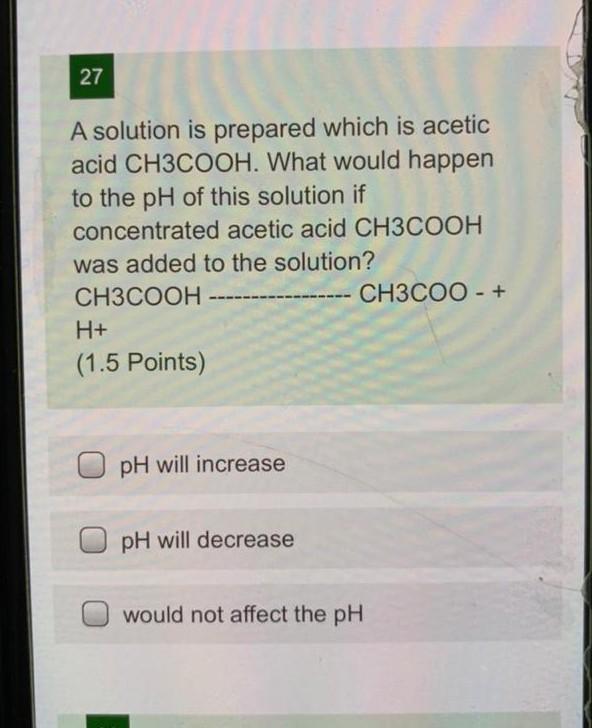
27 A solution is prepared which is acetic acid CH3COOH. What would happen to the pH of this solution if concentrated acetic acid CH3COOH was added to the solution? CH3COOH CH3COO - + H+ (1.5 Points) pH will increase pH will decrease would not affect the pH
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


