Question: 28. Naturally occurring titanium exists as five stable isotopes. Four of the isotopes are 46Ti with a mass of 45.953u(8.0%);47Ti with a mass of 46.952u(7.3%)48Ti

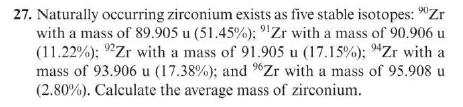
28. Naturally occurring titanium exists as five stable isotopes. Four of the isotopes are 46Ti with a mass of 45.953u(8.0%);47Ti with a mass of 46.952u(7.3%)48Ti with a mass of 47.948u (73.8%); and 49Ti with a mass of 48.948u(5.5%). The average mass of an atom of titanium is 47.9u. Determine the mass of the fifth isotope of titanium. 27. Naturally occurring zirconium exists as five stable isotopes: 90Zr with a mass of 89.905u(51.45%);91Zr with a mass of 90.906u (11.22%);92Zr with a mass of 91.905u(17.15%);94Zr with a mass of 93.906u(17.38%); and 96Zr with a mass of 95.908u (2.80%). Calculate the average mass of zirconium
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


