Question: 2-(a) At some point in the reaction 2A + B + G + 3H, [A] = 0.3629 M. At a time 8.25 min later [A]
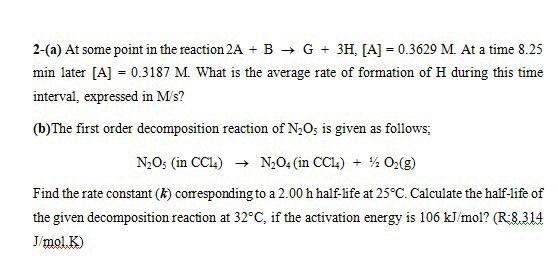
2-(a) At some point in the reaction 2A + B + G + 3H, [A] = 0.3629 M. At a time 8.25 min later [A] = 0.3187 M. What is the average rate of formation of H during this time interval, expressed in M's? (b) The first order decomposition reaction of N2O5 is given as follows: N20s (in CCL) + N204 (in CCL) + % 0 (9) Find the rate constant (k) corresponding to a 2.00 h half-life at 25C. Calculate the half-life of the given decomposition reaction at 32C, if the activation energy is 106 kJ/mol? (R-8.314 J/mol.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


