Question: 3 0 p t s ) You have a circular pipe with 2 0 0 c m long and 1 0 c m 2 cross
You have a circular pipe with long and crosssectional area. You might use the pipe as a reactor in which sucrose is hydrolyzed with the help of an enzyme called sucrase. The pipe is planned to be packed with porous pellets on which sucrase is immobilized. The crosssectional void fraction of the packed pipe reactor is designed to be Total amount of sucrase homogencously dispersed through the porous pellets in the reactor will be mmol. You would like to decide the flow rate of sucrose to obtain conversion of sucrose to products with sucrose as the initial concentration.
You need to know the kinetic information of the reaction. The following kinetic data were oblained in a batch reactor. The concentration of sucrose was calculated from optical rotation measurements.
tableTime hourtableSucrosemM
The rate law corresponding to this reaction is expressed by the MichaelisMenten equation:
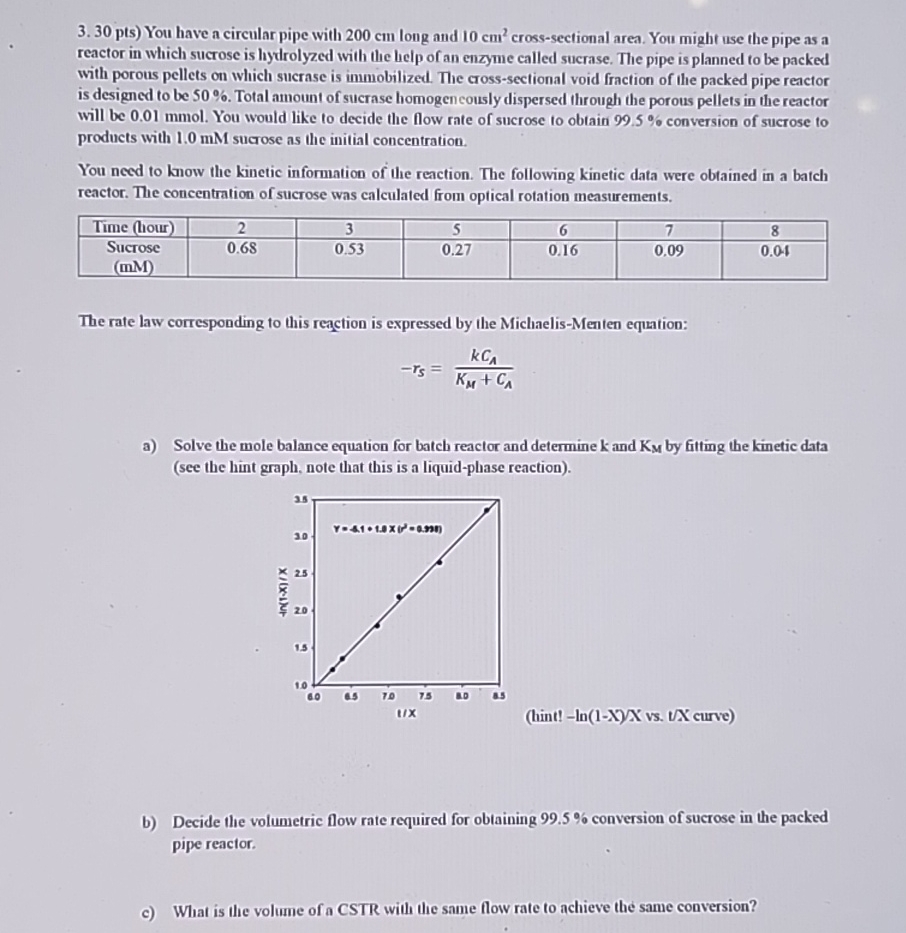
a Solve the mole balance equation for batch reactor and determine and by fitting the kinetic data see the hint graph, note that this is a liquidphase reaction
hint vs curve
b Decide the volumetric flow rate required for obtaining conversion of sucrose in the packed pipe reactor.
c What is the volume of a CSTR wilh the same flow rate to achieve the same conversion?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


