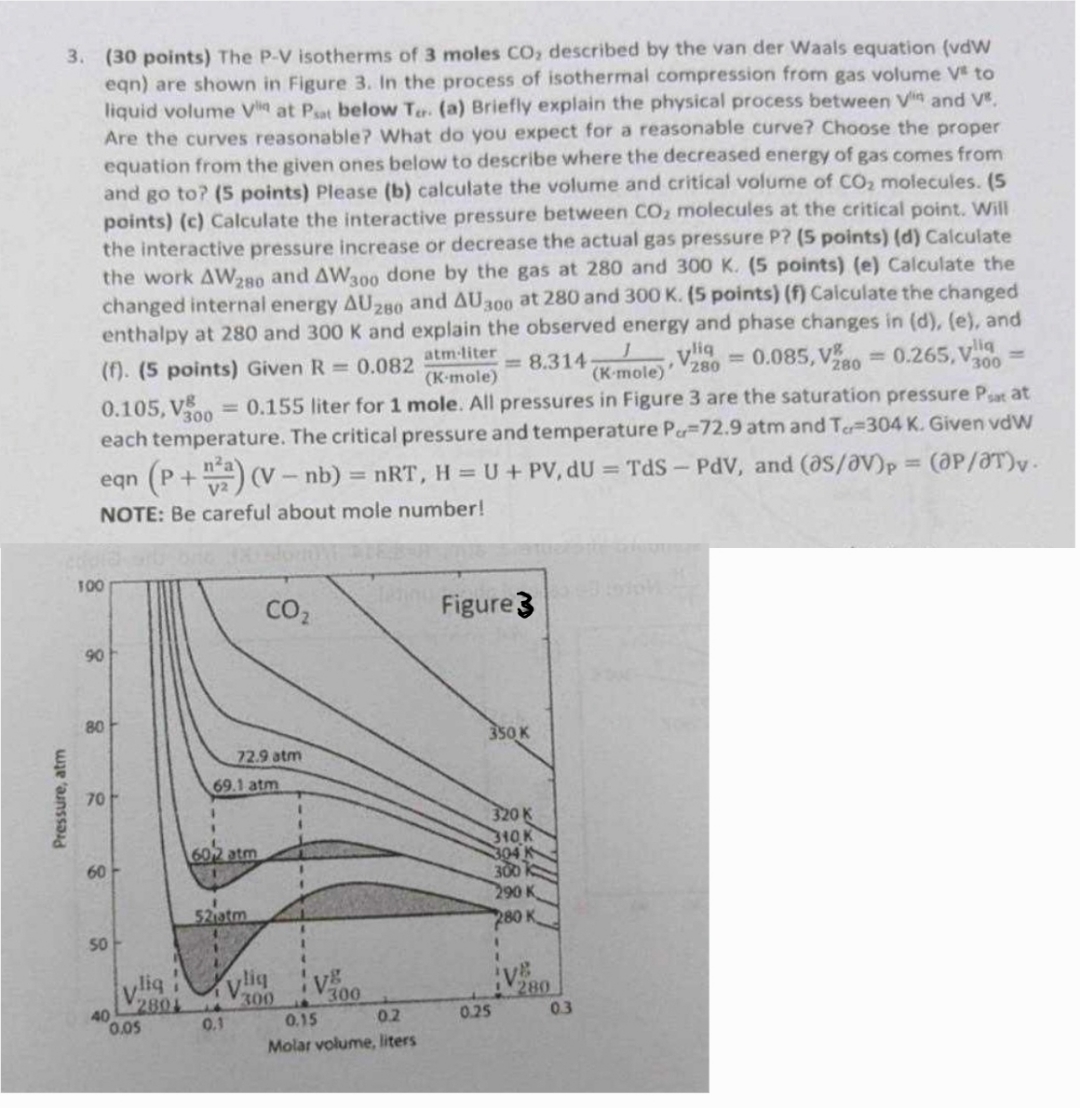
Question: ( 3 0 points ) The P - V isotherms of 3 moles C O 2 described by the van der Waals equation ( vdW
points The PV isotherms of moles described by the van der Waals equation vdW eqn are shown in Figure In the process of isothermal compression from gas volume to liquid volume at below a Briefly explain the physical process between and Are the curves reasonable? What do you expect for a reasonable curve? Choose the proper equation from the given ones below to describe where the decreased energy of gas comes from and go to points Please b calculate the volume and critical volume of molecules. pointsc Calculate the interactive pressure between molecules at the critical point. Will the interactive pressure increase or decrease the actual gas pressure P pointsd Calculate the work and done by the gas at and pointse Calculate the changed internal energy and at and pointsf Calculate the changed enthalpy at and and explain the observed energy and phase changes in de and f points Given liter for mole. All pressures in Figure are the saturation pressure at each temperature. The critical pressure and temperature atm and Given vdW eqn and elVelT NOTE: Be careful about mole number!
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


