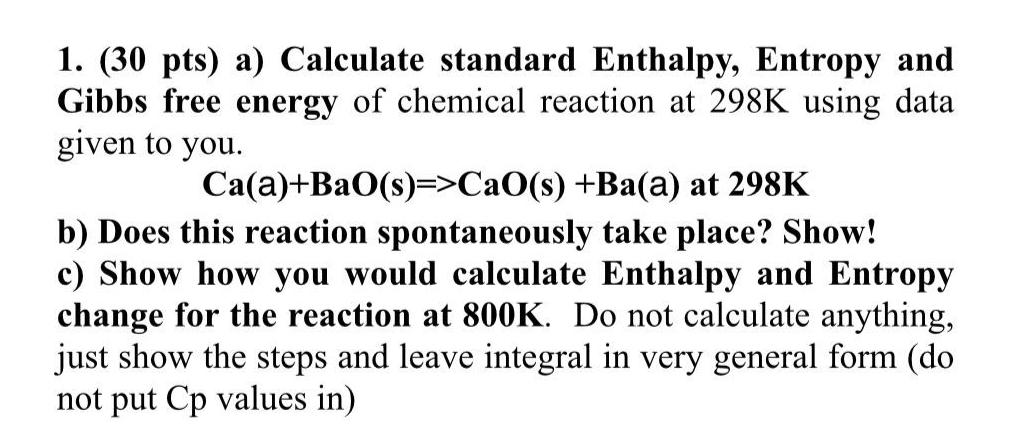
Question: ( 3 0 pts ) a ) Calculate standard Enthalpy, Entropy and Gibbs free energy of chemical reaction at 2 9 8 K using data
pts a Calculate standard Enthalpy, Entropy and Gibbs free energy of chemical reaction at using data given to you.
BaOCaO
b Does this reaction spontaneously take place? Show!
c Show how you would calculate Enthalpy and Entropy change for the reaction at Do not calculate anything, just show the steps and leave integral in very general form do not put values in
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


