Question: Please EXPLAIN and solve EACH / ALL part(s) in Activity #1 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Activity #1!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEWTO CHEMISTRY! I AM A COMPLETE NEWBIE!
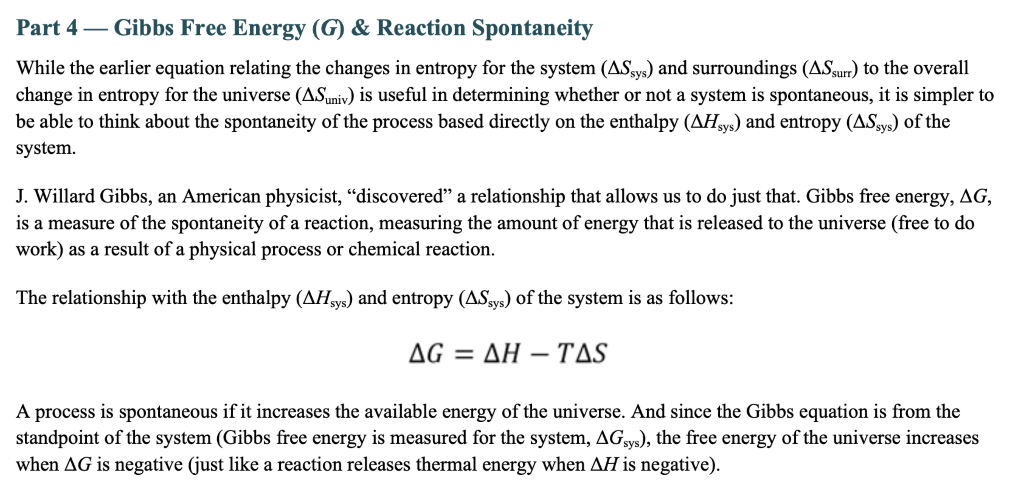

Part 4 - Gibbs Free Energy (G) \& Reaction Spontaneity While the earlier equation relating the changes in entropy for the system (Ssys) and surroundings (Ssurr) to the overall change in entropy for the universe (Suniv) is useful in determining whether or not a system is spontaneous, it is simpler to be able to think about the spontaneity of the process based directly on the enthalpy (Hsys) and entropy (Ssys) of the system. J. Willard Gibbs, an American physicist, "discovered" a relationship that allows us to do just that. Gibbs free energy, G, is a measure of the spontaneity of a reaction, measuring the amount of energy that is released to the universe (free to do work) as a result of a physical process or chemical reaction. The relationship with the enthalpy (Hsys) and entropy (Ssys) of the system is as follows: G=HTS A process is spontaneous if it increases the available energy of the universe. And since the Gibbs equation is from the standpoint of the system (Gibbs free energy is measured for the system, Gsys ), the free energy of the universe increases when G is negative (just like a reaction releases thermal energy when H is negative). - Calculate Gibbs free energy for the following reaction. Then, explain how you could find the temperature range at which the following reaction is spontaneous. Finally, calculate and report the actual temperature range in which the reaction is spontaneous. (Hint: You know enough at this point to answer this question, but if you're stuck, you may need to look ahead to Chapter 13.4 in the subsection titled Temperature-Dependence of Spontaneity.) Reaction: An endothermic reaction absorbs 88.3kJ of energy and undergoes an entropy increase of 238.4J/K at a temperature of 25 C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


