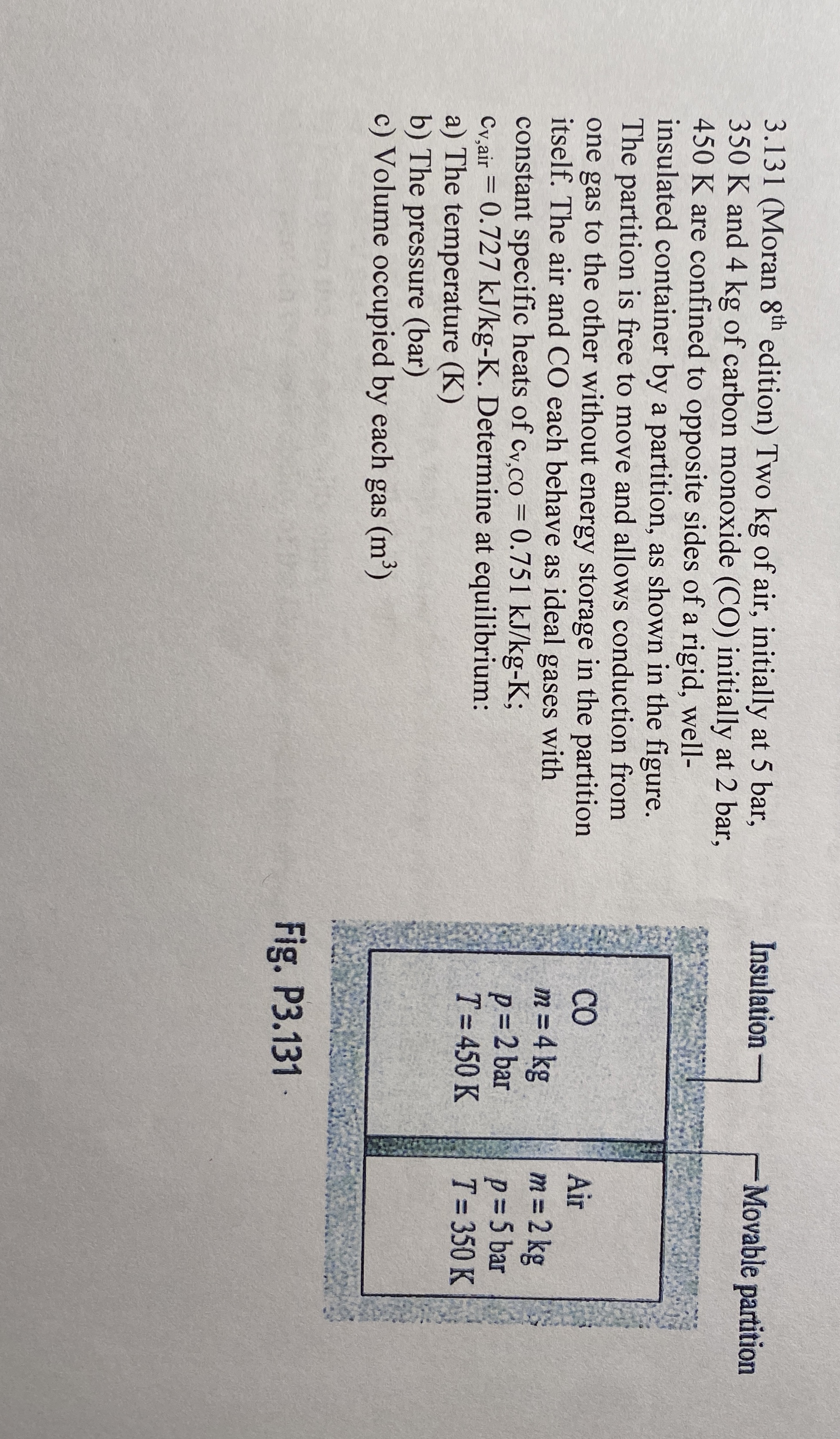
Question: 3 . 1 3 1 ( Moran 8 t h edition ) Two kg of air, initially at 5 bar, 3 5 0 K and
Moran edition Two kg of air, initially at bar,
K and kg of carbon monoxide CO initially at bar,
K are confined to opposite sides of a rigid, well
insulated container by a partition, as shown in the figure.
The partition is free to move and allows conduction from
one gas to the other without energy storage in the partition
itself. The air and CO each behave as ideal gases with
constant specific heats of ;
Determine at equilibrium:
a The temperature K
b The pressure bar
c Volume occupied by each gas
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


