Question: 3 3 QUESTION 2 20 points Save Answer Nitric acid (HNO3) can be produced according to the following reaction between ammonia and oxygen (air is
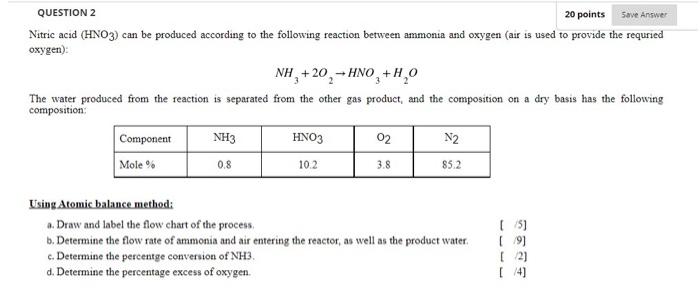
3 3 QUESTION 2 20 points Save Answer Nitric acid (HNO3) can be produced according to the following reaction between ammonia and oxygen (air is used to provide the requried oxygen): NH +20,-HNO + H2O The water produced from the reaction is separated from the other gas product, and the composition on a dry basis has the following composition: Component NH3 HNO3 02 N2 Mole % 10.2 3.8 85.2 0.8 Using Atomic balance method: a. Draw and label the flow chart of the process b. Determine the flow rate of ammonia and air entering the reactor, as well as the product water c. Determine the percentge conversion of NH3. d. Determine the percentage excess of oxygen. (5) (9) [2) [/41
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


