Question: 3. (35 points) (2-component) Carefully plot on the attached graph a binary pressure composition (P-x) diagram at 300F on the basis of the data given
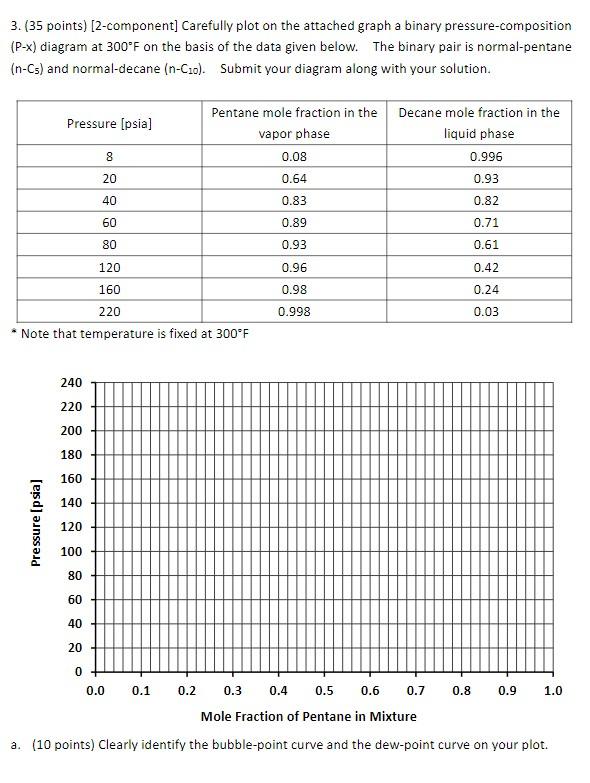

3. (35 points) (2-component) Carefully plot on the attached graph a binary pressure composition (P-x) diagram at 300F on the basis of the data given below. The binary pair is normal-pentane (n-Cs) and normal-decane (n-Co). Submit your diagram along with your solution. Pressure (psia) 8 Pentane mole fraction in the Decane mole fraction in the vapor phase liquid phase 0.08 0.996 0.64 0.93 0.83 0.82 20 40 60 0.89 0.71 80 0.93 0.61 120 0.96 0.42 160 0.98 0.24 220 0.998 0.03 Note that temperature is fixed at 300F 240 220 200 180 160 140 Pressure (psia] 120 100 80 60 40 20 0+ 0.0 0.1 0.8 0.9 1.0 0.2 0.3 0.4 0.5 0.6 0.7 Mole Fraction of Pentane in Mixture a. (10 points) Clearly identify the bubble-point curve and the dew-point curve on your plot. b. (10 points) The critical temperature is approximately 386F (469.7 K) for n-pentane and 652F (617.7 K) for n-decane. On the basis of an extrapolation on your phase diagram, what is the (approximate) vapor pressure of n-pentane at 300F? Indicate your answer clearly in the diagram above. C. (15 points) If a PVT cell contains a mixture of 60 mol% n-pentane and 40 mol% n-decane at 80 psia and 300F, is the fluid an oil, or a gas, or a two-phase system? What is the liquid molar fraction of the fluid in the PVT cell on the basis of the data given? Clearly show your calculation steps (do not use PV Tsim)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


