Question: 3. (43 pts) A and B react to form C by the reaction A + 2B - C in the process below. 100 mol/s A
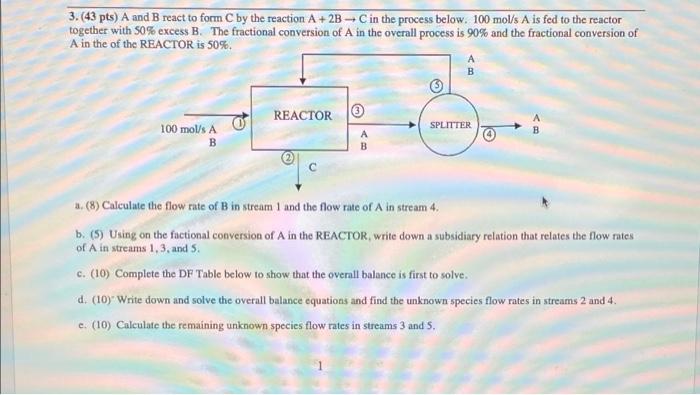
3. (43 pts) A and B react to form C by the reaction A + 2B - C in the process below. 100 mol/s A is fed to the reactor together with 50% excess B. The fractional conversion of A in the overall process is 90% and the fractional conversion of A in the of the REACTOR is 50%. A B REACTOR SPLITTER 100 mol/s A B A 1. (8) Calculate the flow rate of Bin stream 1 and the flow rate of A in stream 4. b. (5) Using on the factional conversion of A in the REACTOR, write down a subsidiary relation that relates the flow rates of A in streams 1,3 and 5. C. (10) Complete the DF Table below to show that the overall balance is first to solve. d. (10) Write down and solve the overall balance equations and find the unknown species flow rates in streams 2 and 4. c. (10) Calculate the remaining unknown species flow rates in streams 3 and S
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


