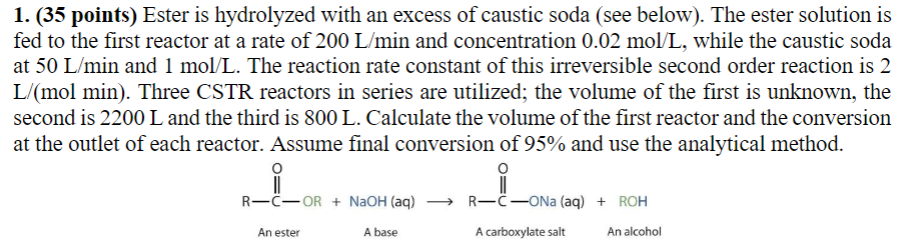
Question: ( 3 5 points ) Ester is hydrolyzed with an excess of caustic soda ( see below ) . The ester solution is fed to
points Ester is hydrolyzed with an excess of caustic soda see below The ester solution is
fed to the first reactor at a rate of and concentration while the caustic soda
at and The reaction rate constant of this irreversible second order reaction is
Three CSTR reactors in series are utilized; the volume of the first is unknown, the
second is and the third is Calculate the volume of the first reactor and the conversion
at the outlet of each reactor. Assume final conversion of and use the analytical method.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


