Question: EG-204 Reactor Design I Tutorial Sheet 4 2022 1. (a) Using mass balance, derive the design performance equations for a CSTR operating with i. ii.
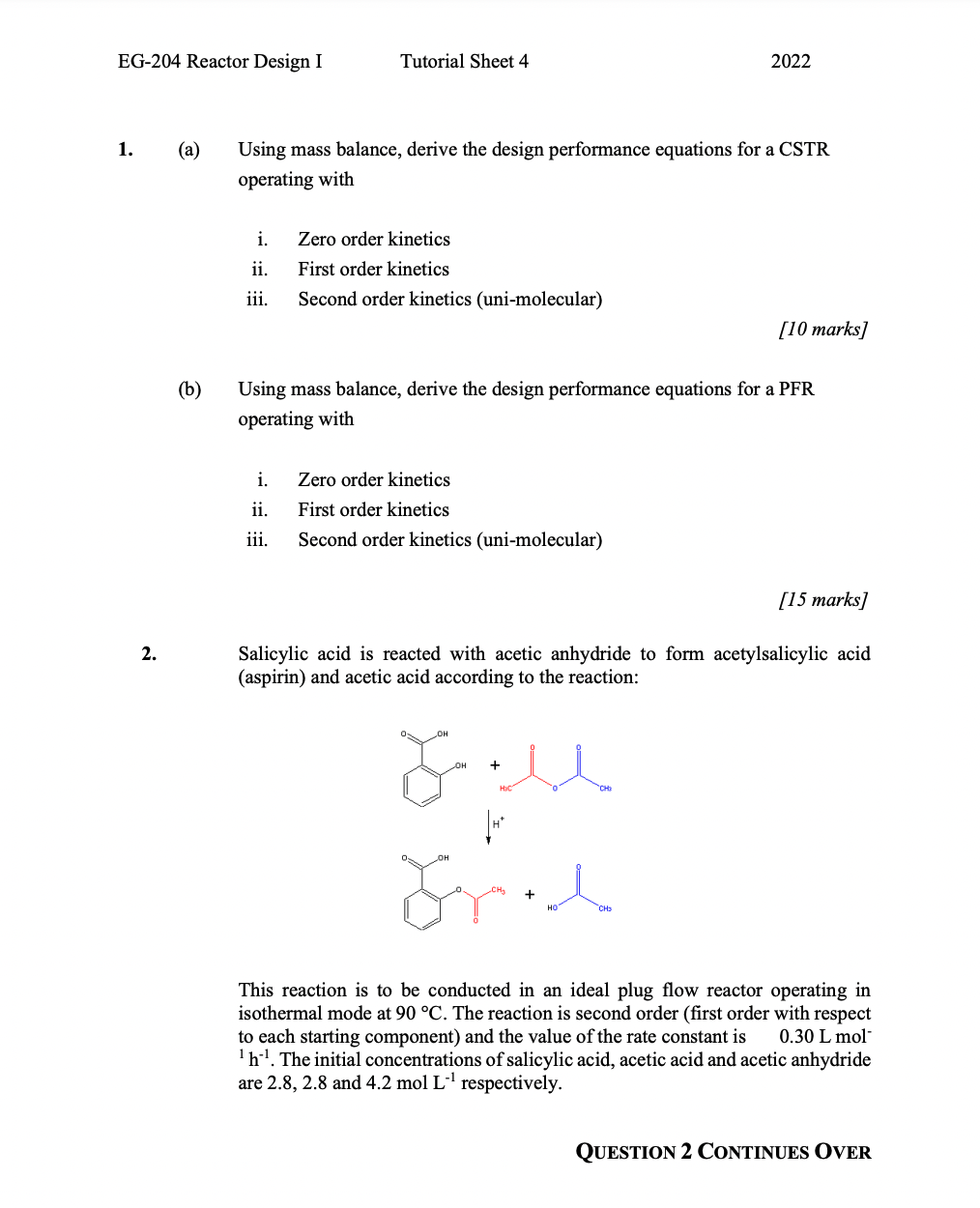
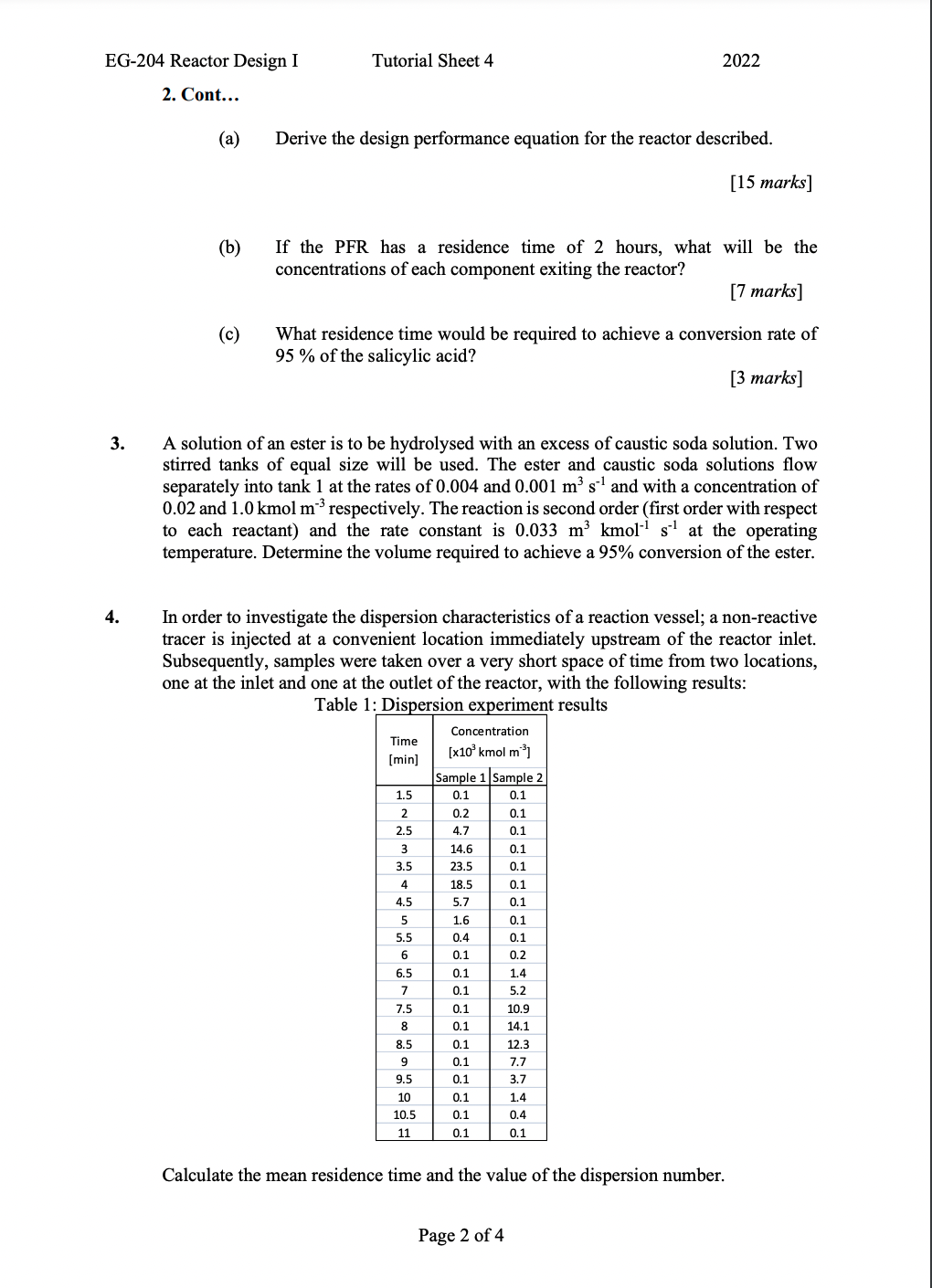
EG-204 Reactor Design I Tutorial Sheet 4 2022 1. (a) Using mass balance, derive the design performance equations for a CSTR operating with i. ii. Zero order kinetics First order kinetics Second order kinetics (uni-molecular) iii. [10 marks] (b) Using mass balance, derive the design performance equations for a PFR operating with i. ii. Zero order kinetics First order kinetics Second order kinetics (uni-molecular) iii. [15 marks] 2. Salicylic acid is reacted with acetic anhydride to form acetylsalicylic acid (aspirin) and acetic acid according to the reaction: + LU Grech CH This reaction is to be conducted in an ideal plug flow reactor operating in isothermal mode at 90 C. The reaction is second order (first order with respect to each starting component) and the value of the rate constant is 0.30 L mol h!. The initial concentrations of salicylic acid, acetic acid and acetic anhydride are 2.8, 2.8 and 4.2 mol L respectively. QUESTION 2 CONTINUES OVER EG-204 Reactor Design I Tutorial Sheet 4 2022 2. Cont... (a) Derive the design performance equation for the reactor described. [15 marks] (b) If the PFR has a residence time of 2 hours, what will be the concentrations of each component exiting the reactor? [7 marks] (C) What residence time would be required to achieve a conversion rate of 95% of the salicylic acid? [3 marks] 3. A solution of an ester is to be hydrolysed with an excess of caustic soda solution. Two stirred tanks of equal size will be used. The ester and caustic soda solutions flow separately into tank 1 at the rates of 0.004 and 0.001 m s1 and with a concentration of 0.02 and 1.0 kmol m' respectively. The reaction is second order (first order with respect to each reactant) and the rate constant is 0.033 m kmoli s at the operating temperature. Determine the volume required to achieve a 95% conversion of the ester. 4. In order to investigate the dispersion characteristics of a reaction vessel; a non-reactive tracer is injected at a convenient location immediately upstream of the reactor inlet. Subsequently, samples were taken over a very short space of time from two locations, one at the inlet and one at the outlet of the reactor, with the following results: Table 1: Dispersion experiment results Time [min] 1.5 2 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 7.5 8 8.5 Concentration [x10 kmol m) Sample 1 Sample 2 0.1 0.1 0.2 0.1 4.7 0.1 14.6 0.1 23.5 0.1 18.5 0.1 5.7 0.1 1.6 0.1 0.4 0.1 0.1 0.2 0.1 1.4 0.1 5.2 0.1 10.9 0.1 14.1 0.1 12.3 0.1 7.7 0.1 3.7 0.1 1.4 0.1 0.4 9 9.5 10 10.5 11 0.1 0.1 Calculate the mean residence time and the value of the dispersion number. Page 2 of 4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


