Question: ( 3 5 % ) , Water initially occupies the left side of the rigid box shown below. It is separated from a vacuum on
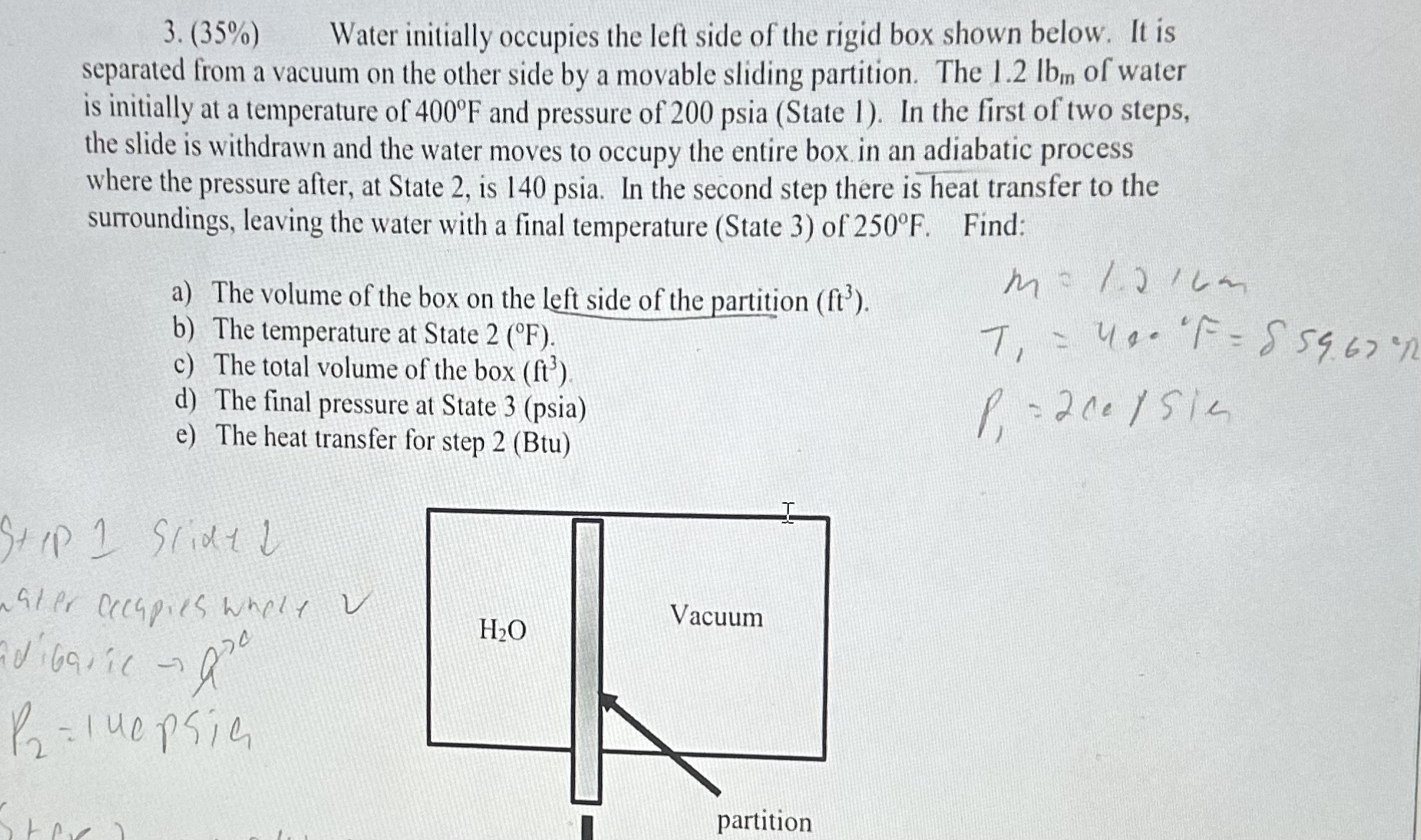
Water initially occupies the left side of the rigid box shown below. It is
separated from a vacuum on the other side by a movable sliding partition. The lb m of water
is initially at a temperature of and pressure of psia State In the first of two steps,
the slide is withdrawn and the water moves to occupy the entire box in an adiabatic process
where the pressure after, at State is psia. In the second step there is heat transfer to the
surroundings, leaving the water with a final temperature State of Find:
a The volume of the box on the left side of the partition
b The temperature at State
c The total volume of the box
d The final pressure at State psia
e The heat transfer for step Btu
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


