Question: 3. A 2.500a gample of vitamin C (aka ascorbic acid C6H8O6;MM=176.12g/mol ) tablet is dissolved in a 200.0mL volumetric flask. A 25.00mL aliquot of the
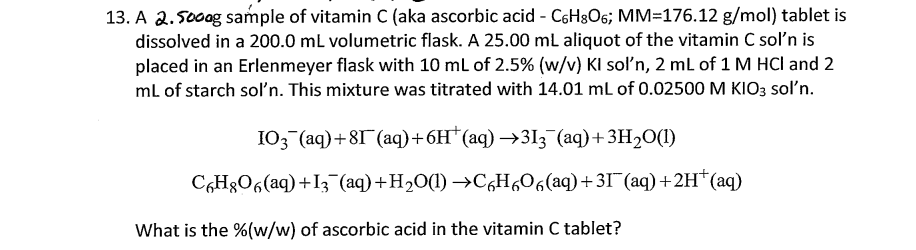
3. A 2.500a gample of vitamin C (aka ascorbic acid C6H8O6;MM=176.12g/mol ) tablet is dissolved in a 200.0mL volumetric flask. A 25.00mL aliquot of the vitamin C sol' n is placed in an Erlenmeyer flask with 10mL of 2.5%(w/v)KI sol'n, 2mL of 1MHCl and 2 mL of starch sol' n. This mixture was titrated with 14.01mL of 0.02500MKIO3 sol' n. IO3(aq)+8I(aq)+6H+(aq)3I3(aq)+3H2O(l)C6H8O6(aq)+I3(aq)+H2O(l)C6H6O6(aq)+3I(aq)+2H+(aq) What is the %(w/w) of ascorbic acid in the vitamin C tablet
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


